Abstract
Lysyl oxidase-like 2 (LOXL2) mediates the crosslinking of extracellular collagen, reflecting qualitative changes in liver fibrosis. This study aimed to validate the utility of serum LOXL2 levels as a predictive biomarker for the development of hepatocellular carcinoma (HCC) in patients with hepatitis C virus (HCV) infection who achieved a sustained virological response (SVR). This retrospective study included 137 patients with chronic HCV infection without history of HCC development and who achieved SVR via direct-acting antiviral therapy. Median LOXL2 levels decreased significantly after SVR achievement (pre-Tx, 2.33 ng/mL; post-Tx, 1.31 ng/mL, p < 0.001). Post-Tx LOXL2 levels, fibrosis-4 index, platelet counts, Wisteria floribunda agglutinin-positive human Mac-2 binding protein levels, and alpha-fetoprotein (AFP) levels were identified as independent predictive factors for post-SVR HCC development in the univariate analysis. The incidence of post-SVR HCC development was significantly higher in patients with post-Tx LOXL2 levels ≥ 2.08 ng/mL and AFP levels ≥ 5.0 ng/mL than in patients with elevated levels of either marker or with lower marker levels. Serum LOXL2 levels can serve as a predictive biomarker for HCC development after achieving SVR. The combination of serum LOXL2 and AFP levels provides robust risk stratification for HCC development after SVR, suggesting an enhanced surveillance strategy.
Similar content being viewed by others
Introduction
Hepatitis C virus (HCV) infection is a global health concern, affecting more than 58 million people worldwide, and a major cause of chronic hepatitis, cirrhosis, and hepatocellular carcinoma (HCC)1. With the development of direct-acting antiviral (DAA) therapy, HCV treatment has made remarkable progress, achieving sustained virological response (SVR) rates of over 95% across all genotypes2,3. Although treatment with DAAs reduces the risk of HCC development and liver disease-related mortality associated with HCV infection, the risk of HCC development after SVR remains between 0.9 and 2.96 per 100 patient-years4,5,6, indicating that HCC still occurs in some patients. As the number of patients who achieve SVR increases, the importance of accurate prediction and appropriate surveillance for HCC after SVR becomes significant.
Several risk factors for HCC development after SVR have been reported7,8,9,10,11, of which liver fibrosis is a significant predictor. Although liver biopsy is considered the gold standard for fibrosis assessment, it has certain limitations, including sampling variability and the potential for severe complications12. Consequently, the demand for non-invasive biomarkers for the evaluation of liver fibrosis and prediction of HCC development after SVR has grown13.
Lysyl oxidase-like 2 (LOXL2) is an enzyme that catalyzes collagen crosslinking in the extracellular matrix (ECM)14,15. The liver collagens modified by LOXL2 become tightly crosslinked and difficult to solubilize. The LOX family consists of LOX and LOXL1-4; LOXL2 is expressed in the liver and other organs, including the breast, lungs, gastrointestinal tract, and kidneys, where it has various functions16. It is associated with various pathogenesis-related processes, such as post-translational modification of ECM collagens, epithelial-mesenchymal transition (EMT), angiogenesis, and cancer progression or metastasis17,18,19,20,21.
In general, liver fibrosis, from chronic hepatitis to early-stage cirrhosis, ameliorates to some extent after achieving SVR with interferon (IFN) or DAA22,23,24,25,26,27 treatment; however, in some cases where insoluble fibers are already deposited, fibrosis is highly resistant to fibrinolysis, and significant improvement is not achieved after SVR, thus increasing the risk for HCC28,29. Markers such as LOXL2 that specifically focus on the "quality" or "solubility", rather than the severity, of fibrosis can also be valuable for predicting HCC after achieving SVR.
Considering cost-effectiveness, the modality and frequency of HCC screening should be individually optimized according to personal risk profiles9,30. Establishing a valid HCC risk assessment method using non-invasive biomarkers to provide a personalized follow-up system is essential. The aim of this study was to verify the utility of LOXL2 as a predictive biomarker for HCC development after SVR in patients with HCV, and to use it to stratify risk, enabling an efficient surveillance strategy.
Results
Patient characteristics
The baseline characteristics of the 137 patients are summarized in Table 1. The median age was 67 years, 73 (53.3%) patients were male, 65 (47.4%) had a history of an IFN-based regimen, 6 (4.4%) consumed 20 g or more of alcohol per day, and 22 (16.1%) had diabetes mellitus. The median observation period was 4.8 years (0.2–7.3). The median alpha-fetoprotein (AFP) and LOXL2 levels in the serum were significantly higher in the pre-treatment (pre-Tx) than in the post-treatmant (post-Tx) samples (AFP; 5.8 and 3.5 ng/mL, LOXL2; 2.33 and 1.31 ng/mL, respectively). The median LOXL2 level in the patients with HCV was significantly higher than that in healthy volunteers (N = 13) (2.33 vs. 1.39 ng/mL, p = 0.002; Supplementary Fig. 1).
Correlation of serum LOXL2 levels with liver fibrosis or tumor markers
We investigated whether serum LOXL2 levels correlated with various markers for liver fibrosis or tumor. Pre- and post-Tx LOXL2 levels did not show strong correlations with platelet counts, Wisteria floribunda agglutinin-positive human Mac-2 binding protein (WFA+-M2BP) levels, fibrosis-4 (FIB-4) index, albumin-bilirubin (ALBI), or AFP levels in the serum (Supplementary Fig. 2).
HCC development in patients who achieved SVR
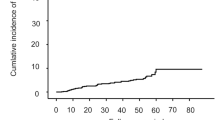
During the observation period, 20 of the 137 patients (14.6%) developed post-SVR HCC. Detail backgrounds of patients developed post-SVR HCC were shown in Supplementary Table 1. The cumulative post-SVR incidence of HCC at 1, 3, and 5 years was 3.0, 11.0, and 16.3%, respectively (Fig. 1). Patients with post-SVR HCC were significantly older (p = 0.034) and had lower platelet counts (p = 0.032), higher AFP levels (p < 0.001), higher WFA+-M2BP levels (p = 0.047), and higher FIB-4 index (p = 0.012) post-Tx than those who did not develop post-SVR HCC. Pre- and post-Tx serum LOXL2 levels in the patients with post-SVR HCC were also higher than in those without, but the difference was not statistically significant (Table 2).
Predictive factors associated with HCC development after achieving SVR
Univariate analysis identified factors that predicted the risk for HCC development after achieving SVR. Cox regression analysis was performed on six backgrounds (age, sex, body mass index [BMI], alcohol intake, presence of diabetes mellitus, and history of IFN treatment) and eight pre- or post-Tx variables (platelet counts, aspartate aminotransferase [AST], alanine aminotransferase [ALT], AFP, and WFA+-M2BP levels, FIB-4 index, ALBI, and LOXL2 levels). The cut-off values for these factors, except ALBI and LOXL2, were set according to previous reports7,31,32,33,34,35,36,37,38,39. The cut-off values for ALBI and LOXL2 were set as − 2.27, which distinguishes a modified (m)ALBI grade 2a from 2b40,41,42, and 2.08, based on a receiver operating characteristic (ROC) curve, respectively (Supplementary Fig. 3).
Among background factors, age ≥ 65 years (hazard ratio [HR] 3.117, 95% confidence interval [CI] = 1.190–8.164, p = 0.021) and diagnosis with diabetes mellitus (HR 2.624, 95% CI = 1.007–6.840, p = 0.048) were identified as risk factors for post-SVR HCC development. Regarding pre-Tx factors, platelet count < 120 × 109/L (HR 2.453, 95% CI = 1.019–5.910, p = 0.045), AFP level ≥ 10.0 ng/mL (HR 3.081, 95% CI = 1.268–7.484, p = 0.013), WFA+-M2BP level ≥ 2.8 (cut-off index, COI) (HR 3.138, 95% CI = 1.204–8.181, p = 0.019), and FIB-4 index ≥ 3.25 (HR 3.604, 95% CI = 1.382–9.400, p = 0.009) were identified. Platelet count < 120 × 109/L (HR 3.150, 95% CI = 1.302–7.622, p = 0.011), AFP level ≥ 5.0 ng/mL (HR 4.486, 95% CI = 1.781–11.30, p = 0.001), WFA+-M2BP level ≥ 1.0 COI (HR 3.383, 95% CI = 1.130–10.13, p = 0.029), FIB-4 index ≥ 2.9 (HR 2.703, 95% CI = 1.077–6.783, p = 0.034), and LOXL2 level ≥ 2.08 ng/mL (HR 2.939, 95% CI = 1.203–7.178, p = 0.018) were identified among post-Tx factors (Table 3).
Multivariate analysis was performed to investigate confounding factors between serum LOXL2 levels and other factors. Combinations of serum LOXL2 levels with serum AFP level, platelet count, age, and presence of diabetes mellitus, were identified as independent risk factors for post-SVR HCC development (Table 4). In contrast, when serum LOXL2 levels were combined with the FIB-4 index or WFA+-M2BP levels, only LOXL2 levels were extracted.
Stratification for the risk of HCC development after achieving SVR with predictive factors
The cumulative post-SVR incidence rate of HCC was examined using the Kaplan–Meier method. Significant differences in the post-SVR incidence rate of HCC were observed when the patients were divided into two groups by either LOXL2 (< 2.08 vs. ≥ 2.08 ng/mL) or AFP (< 5.0 vs. ≥ 5.0 ng/mL) post-Tx levels (p = 0.013 and < 0.001, respectively).
In the comparison of patients with post-Tx LOXL2 levels < 2.08 versus ≥ 2.08 ng/mL, the cumulative incidence rates of HCC were 1.9% versus 7.1% at 1 year, 9.1% versus 18.2% at 3 years, and 11.9% versus 30.5% at 5 years. When comparing patients with post-Tx AFP levels < 5.0 versus ≥ 5.0 ng/mL, the cumulative incidence rates of HCC were 0% versus 9.5% at 1 year, 5.8% versus 22.4% at 3 years, and 9.3% versus 31.7% at 5 years, respectively (Fig. 2A,B).
Cumulative Incidence of HCC in Patients who Achieved SVR Stratified according to Serum LOXL2 and AFP Levels. The cumulative post-SVR incidence of HCC development stratified by (A) post-Tx serum LOXL2 levels and (B) post-Tx serum AFP levels was assessed using the Kaplan–Meier method. (A) The red and black lines indicate post-Tx LOXL2 levels stratified by ≥ 2.08 and < 2.08 ng/mL, respectively. The incidence rate in patients with LOXL2 levels ≥ 2.08 ng/mL was significantly higher than that in patients with lower LOXL2 levels (p = 0.013, log-rank test). (B) The red and black lines indicate post-Tx AFP levels stratified by ≥ 5.0 and < 5.0 ng/mL, respectively. The incidence rate in patients with AFP levels ≥ 5.0 ng/mL was significantly higher than that in patients with lower AFP levels (p < 0.001, log-rank test). Abbreviations: AFP, alpha-fetoprotein; HCC, hepatocellular carcinoma; LOXL2, lysyl oxidase-like 2; post-Tx, post-treatment; SVR, sustained virological response.
Stratifications by post-Tx levels of platelet count (< 120 vs. ≥ 120 × 109/L), WFA+-M2BP (< 1.0 vs. ≥ 1.0 COI), and FIB-4 index (< 2.9 vs. ≥ 2.9) also revealed significant difference in the post-SVR incidence rate of HCC, while no significant difference was observed with ALBI (< -2.27 vs. ≥ -2.27) (Supplementary Fig. 4).
To stratify patients for risk of post-SVR HCC development, we developed a scoring system using post-Tx LOXL2 and AFP levels (LOXL2-AFP or LA score). The number of risk factors was counted and directly used for risk stratification. Ten patients (7.3%) achieved 2 points in the LA scoring system (high-risk group; levels of LOXL2 ≥ 2.08 ng/mL and of AFP ≥ 5.0 ng/mL); 52 patients (38.0%) 1 point (medium-risk group; either LOXL2 levels ≥ 2.08 ng/mL or AFP levels ≥ 5.0 ng/mL); and the remaining 75 patients (54.7%) scored 0 points (low-risk group; levels of LOXL2 < 2.08 ng/mL and of AFP < 5.0 ng/mL) (Fig. 3). Based on the LA system, the cumulative post-SVR incidence of HCC was highest for the 2-point group, next for the 1-point group, and minimum for the 0-point group (p < 0.001). The cumulative post-SVR incidence of HCC at 1, 3, and 5 years was 22.2%, 33.3%, and 60.0% for the 2-point group; 3.8%, 16.1%, and 20.9% for the 1-point group; and 0%, 4.5%, and 7.1% for the 0-point group, respectively.
Stratification for the Risk of HCC Development after SVR by Combining LOXL2 and AFP Levels. The cumulative post-SVR incidence of HCC development was significantly stratified (p < 0.001) by the LA score. The green, red, and black lines represent the 2-point group in the LA scoring system (high-risk), the 1-point group (medium-risk), and the 0-point group (low-risk), respectively. The cumulative post-SVR incidence of HCC development was assessed using the Kaplan–Meier method and log-rank test. Abbreviations: AFP, alpha-fetoprotein; HCC, hepatocellular carcinoma; LA score, LOXL2-AFP score; LOXL2, lysyl oxidase-like2; SVR, sustained virological response.
Discussion
The present study demonstrates the utility of serum LOXL2 levels in predicting HCC development after achieving SVR. Given that liver fibrosis is one of the significant factors in post-SVR HCC development, various markers that reflect the severity of fibrosis, such as the FIB-4 index, WFA+‐M2BP levels, and platelet counts, have been extensively investigated and reported as predictors7,34,36,37,38,39,43. Degradation of the deposited fibers in the ECM occurs in patients who have achieved SVR following DAA therapy25,26,27. The wide variation in the rate and extent of fiber degradation among patients who achieve SVR implies that fiber insolubility or stability affects post-SVR HCC development. Therefore, we specifically focused on the association between post-translational fiber modifications and post-SVR HCC development, and then validated the effectiveness of the novel parameter LOXL2.
In our initial investigation, we characterized the serum LOXL2 levels as a clinical marker. The concentration of serum LOXL2 was elevated in patients infected with HCV compared to that in healthy individuals, and it rapidly decreased following HCV clearance, suggesting a parallel with the resolution of inflammation. This pattern was also observed for the FIB-4 index and WFA+-M2BP levels, which decreased significantly after SVR was achieved. LOXL2 is induced via various pathways. Notably, the promoter region of LOXL2 contains the SMAD binding sequence, indicating that LOXL2 expression increases in parallel with virus-induced inflammation and subsequent TGF-β expression17. LOXL2 expression is also triggered by the hypoxia/hypoxia-inducible factor-1 (HIF-1) pathway, or by ECM stiffness followed by the activation of integrins, c-Jun N-terminal kinase (JNK)/c-Jun, or MEK1/2-ERK1/217,44.
Besides its diverse induction pathways, LOXL2 exhibits a variety of functions other than ECM cross-linking, including EMT regulation, cell migration, and angiogenesis16,17,18,19,20,21. Surprisingly, two forms of LOXL2 have been described: one secreted into the extracellular space, and the other, which features a modified N-terminus, retained within cells. The function of the latter has not been fully elucidated, but it appears to perturb tumor-related pathways and possibly regulate cell death or proliferation within hepatocytes19,45,46,47. Considering its diverse induction pathways and functions, it is more reasonable to regard LOXL2 levels as a pathophysiological biomarker along a novel axis, rather than as a marker in the context of liver fibrosis.
We demonstrated that serum post-Tx LOXL2 levels ≥ 2.08 ng/mL is an independent factor for predicting HCC development after achieving SVR. We also confirmed that known fibrosis markers at the pre-Tx period (platelet count < 120 × 109/L, AFP levels ≥ 10.0 ng/mL, FIB-4 index ≥ 3.25, WFA+-M2BP ≥ 2.8 COI) or post-Tx (platelet count < 120 × 109/L, AFP ≥ 5.0 ng/mL, FIB-4 index ≥ 2.9, WFA+-M2BP ≥ 1.0 COI) were significant. Although it has not been conclusively determined whether pre- or post-Tx assessment is more advantageous, our results indicated that post-Tx assessment tended to have higher HRs in the univariate analysis than pre-Tx, except for the FIB-4 index. Sato et al. reported similar results regarding WFA+-M2BP levels, supporting the notion that post-Tx evaluation is a more robust predictive factor for post-SVR HCC development43. In predicting HCC development after achieving SVR, it might be crucial to evaluate post-Tx risk factors that are not affected by inflammation.
Serum LOXL2 levels between the patients with HCC and those without HCC were not statistically different in Table 2; however, when utilizing the most suitable cut-off value calculated from the ROC curve for predicting HCC, serum LOXL2 level was found to be a significant predictive factor (Table3). The reason for this discrepancy is considered to be that the mean value was influenced by some cases, especially in the patients without HCC, that deviate from the mean.
Lastly, we identified high serum levels of LOXL2 (≥ 2.08 ng/mL) and AFP (≥ 5.0 ng/mL) as independent risk factors of HCC development after achieving SVR. The underlying mechanism for post-SVR HCC development is multifactorial and includes persistent metabolic abnormalities after virus clearance, damage resulting from other liver diseases, and severe liver fibrosis without degradation48,49. Therefore, predictions using a single marker that reflects a single pathophysiological characteristic are challenging. We stratified patients to further differentiate the risk using serum levels of LOXL2 ≥ 2.08 ng/mL and of AFP ≥ 5.0 ng/mL. We established the LA score, which comprises post-Tx serum LOXL2 and AFP levels, and validated performance in our cohort. The LA score identified three groups significantly different regarding the risk of HCC development after achieving SVR: a high-risk 2-point group (with levels of LOXL2 ≥ 2.08 ng/mL and of AFP ≥ 5.0 ng/mL), a medium-risk 1-point group (levels of either LOXL2 ≥ 2.08 ng/mL or of AFP ≥ 5.0 ng/mL), and a low-risk 0-point group (lower LOXL2 and AFP levels). Previous studies have also succeeded in improving the accuracy of HCC prediction after achieving SVR by combining multiple markers50,51,52.
Interestingly, the combinations of serum LOXL2 levels ≥ 2.08 ng/mL with a platelet count < 120 × 109/L, age ≥ 65 years, or a diagnosis with diabetes mellitus were identified as independent risk factors in the multivariate analysis, but effective risk stratification was not achieved. Furthermore, when the serum LOXL2 level was combined with the FIB-4 index or WFA+-M2BP level, only LOXL2 was extracted as a predictive factor. As expected, stratified analysis using the combination of LOXL2 level and FIB-4 index or WFA+-M2BP level did not achieve effective risk stratification (data not shown). The level of serum AFP is an excellent predictor of HCC development after achieving SVR, particularly in terms of specificity rather than sensitivity31,53. Rocha et al. have reported the efficacy of this parameter in predicting HCC after SVR, suggesting that further reinforcement is necessary in conjunction with other markers53. In the current results, serum LOXL2 levels showed superior sensitivity. One reason why consideration of serum AFP levels with LOXL2 levels allows for effective stratification appears to be that the serum AFP and LOXL2 levels may mutually complement their weaknesses.
In recent clinical trials, simtuzumab, a monoclonal antibody against LOXL2, failed to effectively resolve fibrosis in patients with non-alcoholic steatohepatitis (NASH)54. This negative outcome could be related to an inefficient antibody product rather than LOXL2 not being a relevant target for antifibrotic therapy55. Since LOXL2 is a multifunctional molecule associated with various biological processes, efforts to target it for diagnosis or treatment are still ongoing20,55,56,57.
The present study has certain limitations. First, the lack of a validation cohort made it impossible to assess the reproducibility of the results. Second, a small number of patients even in the 0-point group based on the LA score developed post-SVR HCC, indicating that the proposed system did not identify individuals at zero risk of developing the disease. This issue is crucial, because it is clinically significant to identify patients with no risk of developing post-SVR HCC and thus require no further surveillance. Addressing this limitation requires further investigation. Lastly, the retrospective design and potential selection bias in our study should not be neglected. Given the limited number of patients with HCC who achieved SVR, we were unable to use Cox proportional hazards analysis with multiple factors to extract the strongest predictors. Regarding metabolic disorders, our study confirmed that a pre-Tx diagnosis with diabetes mellitus was an independent risk factor along with serum LOXL2 levels; however, the number of patients who were positive for both parameters was too small (N = 5) for evaluation. Metabolic disorders have been actively investigated as factors associated with HCC development after achieving SVR and promising results have been reported58,59,60. The use of metabolic factors in predicting post-SVR HCC development remains to be addressed. Our cohort exhibited potential biases due to the restriction of cases to genotype 1 and a higher proportion of cases with advanced fibrosis. The latter was attributed to the approval of daclatasvir (DCV)/asunaprevir (ASV) in 2014 as the first interferon-free treatment in Japan, which was used for many cases with advanced fibrosis ineligible for interferon therapy. Furthermore, our emphasis on managing high-risk cases with advanced fibrosis may have led to the loss of follow-up for some cases with relatively mild fibrosis after achieving SVR. It is speculated that these biases may account for the increased incidence of HCC development after achieving SVR in our cohort compared to recent reports8,61.
To address these issues, more cases are necessary to conduct a larger-scale prospective study.
Conclusions
This study identified post-Tx serum LOXL2 levels as a valuable predictive marker for HCC development after achieving SVR. Additionally, a new scoring system that combines post-Tx serum LOXL2 and AFP levels allowed stratification of patients according to their risk of post-SVR HCC incidence. Future prospective studies with larger cohorts are required to validate our predictive model for HCC development after achieving SVR.
Methods
Patients
This retrospective multicenter cohort study conducted by the DAA Study in Hamamatsu group (DASH study group), involved 9 institutions (Hamamatsu University Hospital, Shimada General Medical Center, Seirei Hamamatsu General Hospital, Hamamatsu Medical Center, Iwata City Hospital, Shizuoka City Shizuoka Hospital, Minoru Medical Clinic, Tamakoshi Clinic, Elm Medical Clinic). The study was approved by the Ethics Committee of Hamamatsu University School of Medicine (approval number, 23-224). The study protocols conformed to the ethical guidelines of the Declaration of Helsinki. All patients provided written informed consent. Patients diagnosed with serogroup 1 chronic HCV infection who initiated IFN-free DAA regimens (DCV/ASV or sofosbuvir [SOF]/ledipasvir [LDV]) between August 2014 and December 2016 at nine affiliated institutions were enrolled. SVR was defined as absence of detectable HCV RNA at 24 weeks after treatment completion. No relapse of viremia was observed after 24 weeks in the patients who achieved SVR. Serum samples from healthy volunteers were also collected during the study period.
The exclusion criteria were as follows: (1) absence of sufficiently stored serum samples; (2) coinfection with either hepatitis B virus (HBV) or human immunodeficiency virus; (3) history of other chronic liver diseases (autoimmune hepatitis, primary biliary cholangitis, hemochromatosis, or Wilson’s disease); (4) history of HCC development at enrollment; (5) HCC development in the period up to 24 weeks after treatment completion, detected using ultrasonography (US), contrast-enhanced computed tomography (CT), or contrast-enhanced magnetic resonance imaging (MRI); (6) serum LOXL2 levels (see below) under the lower limit of quantification (LLQ). After exclusions, the data from 137 patients were retrospectively analyzed to identify risk factors for HCC development after achieving SVR (Fig. 4).
Study Flowchart Illustrating the Patient Selection Process. The development of HCC was studied in patients with HCV who achieved SVR via DAA therapy. Abbreviations: ASV, asunaprevir; DAA, direct-acting antiviral; DCV, daclatasvir; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; LDV, ledipasvir; LLQ, lower limit of quantification; LOXL2, lysyl oxidase-like 2; SOF, sofosbuvir; SVR, sustained virological response.
Laboratory tests
Blood samples were collected on the day before the initiation of DAA treatment (pre-Tx) and 24 weeks after treatment completion (post-Tx). Serum samples were separated and stored at − 80 °C. Hematological and biochemical parameters, including platelet counts, levels of AST, ALT, total bilirubin, gamma-glutamyl transpeptidase (GGT), albumin, and AFP, and HCV serogroup were measured at each institution using standard techniques. Measurement of the WFA+-M2BP levels were performed by the contract laboratory (SRL Inc, Tokyo, Japan) using stored serum. Serum HCV-RNA titers were measured using the Roche COBAS TaqMan test (LLQ; 1.2 log10 IU/mL; Roche Molecular Diagnostics, CA) or AccuGene m-HCV (LLQ; 1.1 log10 IU/mL; Abbott Japan, Tokyo, Japan). The FIB-4 index and ALBI scores were calculated and used as surrogate indicators of liver fibrosis and function, respectively62,63.
Measurement of human LOXL2 levels
Stored pre-Tx and post-Tx serum samples were used to quantify LOXL2 levels using an enzyme-linked immunosorbent assay (ELISA) kit (Cloud-Clone Corp, TX). The detection range was 0.156–10 ng/mL.
Surveillance and diagnosis of HCC
All patients were followed-up at intervals of 1–6 months by measuring their biochemical and virological values and blood counts. Imaging examinations (US, contrast-enhanced CT, or contrast-enhanced MRI) were performed at least once every 6 months. A diagnosis of HCC development was based on findings of typical vascular patterns on contrast-enhanced CT or MRI.
Statistical analysis
All statistical analyses were performed using SPSS ver. 25.0 (SPSS, Chicago, IL, USA), GraphPad Prism (ver. 9.0; GraphPad Software, San Diego, CA), and EZR (ver 1.60; Saitama Medical Center, Jichi Medical University, Saitama, Japan). The data are presented as medians and ranges, or numbers and percentages. The chi-square and Mann–Whitney U tests were used to evaluate categorical data and continuous variables, respectively. The Wilcoxon signed-rank test was used to assess differences between the two paired groups. The Cox proportional hazards model was used to evaluate the association between each variable and HCC development (univariate and multivariate analyses). The Kaplan–Meier method and log-rank test were used to assess the cumulative incidence of HCC in each group. The correlation between the two variables was evaluated using Pearson’s correlation coefficient. p < 0.05 was considered to indicate statistical significance.
Data availability
The datasets generated and/or analyzed during this study are available from the corresponding author on reasonable request.
Abbreviations
- AFP:
-
Alpha-fetoprotein
- ALBI:
-
Albumin-bilirubin
- ALT:
-
Alanine aminotransferase
- ANOVA:
-
Analysis of variance
- ASV:
-
Asunaprevir
- AST:
-
Aspartate aminotransferase
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- COI:
-
Cut-off index
- CT:
-
Computed tomography
- DAA:
-
Direct-acting antiviral
- DCV:
-
Daclatasvir
- ECM:
-
Extracellular matrix
- ELISA:
-
Enzyme-linked immunosorbent assay
- EMT:
-
Epithelial-mesenchymal transition
- ERK1/2:
-
Extracellular signal-regulated kinase 1/2
- FIB-4:
-
Fibrosis-4
- GGT:
-
Gamma-glutamyl transpeptidase
- HIF-1:
-
Hypoxia-inducible factor-1
- HBV:
-
Hepatitis B virus
- HCC:
-
Hepatocellular carcinoma
- HCV:
-
Hepatitis C virus
- HR:
-
Hazard ratio
- IFN:
-
Interferon
- Jnk:
-
C-jun N-terminal kinase
- LA score:
-
LOXL2-AFP score
- LDV:
-
Ledipasvir
- LLQ:
-
Lower limit of quantification
- LOX:
-
Lysyl oxidase
- LOXL2:
-
Lysyl oxidase-like 2
- MEK1/2:
-
Mitogen-activated protein kinase 1/2
- MRI:
-
Magnetic resonance imaging
- NASH:
-
Non-alcoholic steatohepatitis
- pre-Tx:
-
Pre-treatment
- post-Tx:
-
Post-treatment
- ROC:
-
Receiver operating characteristic
- SOF:
-
Sofosbuvir
- SVR:
-
Sustained virological response
- US:
-
Ultrasonography
- WFA+-M2BP:
-
Wisteria floribunda agglutinin-positive human Mac-2 binding protein
References
World Health Organization (WHO) Hepatitis C. World Health Organization (WHO) Hepatitis C. https://www.who.int/news-room/fact-sheets/detail/hepatitis-c.
Chou, R. et al. Screening for hepatitis C virus infection in adolescents and adults: Updated evidence report and systematic review for the US preventive services task force. JAMA https://doi.org/10.1001/jama.2019.20788 (2020).
Nyberg, L. et al. Real-world value of direct-acting antivirals for hepatitis C at Kaiser Permanente Southern California. Am. J. Managed Care 29, e299–e306 (2023).
Ioannou, G. N., Green, P. K. & Berry, K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J. Hepatol. 68, 25–32 (2018).
Kanwal, F. et al. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology 153, 996-1005.e1 (2017).
Waziry, R. et al. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: A systematic review, meta-analyses, and meta-regression. J. Hepatol. 67, 1204–1212 (2017).
Ideno, N. et al. Fib-4 index predicts prognosis after achievement of sustained virologic response following direct-acting antiviral treatment in patients with hepatitis C virus infection. Eur. J. Gastroenterol. Hepatol. 35, 219–226 (2023).
Semmler, G. et al. HCC risk stratification after cure of hepatitis C in patients with compensated advanced chronic liver disease. J. Hepatol. 76, 812–821 (2022).
Ahumada, A., Rayón, L., Usón, C., Bañares, R. & Alonso Lopez, S. Hepatocellular carcinoma risk after viral response in hepatitis C virus-advanced fibrosis: Who to screen and for how long?. World J. Gastroenterol. 27, 6737–6749 (2021).
Ioannou, G. N. et al. Assessment of a deep learning model to predict hepatocellular carcinoma in patients with hepatitis C cirrhosis. JAMA Netw. Open 3, e2015626 (2020).
Lleo, A. et al. Predictors of hepatocellular carcinoma in HCV cirrhotic patients treated with direct acting antivirals. Dig. Liver Dis. 51, 310–317 (2019).
Bravo, A. A., Sheth, S. G. & Chopra, S. Liver biopsy. N. Engl. J. Med. 344, 495–500 (2001).
Barnard Giustini, A., Ioannou, G. N., Sirlin, C. & Loomba, R. Review article: Available modalities for screening and imaging diagnosis of hepatocellular carcinoma-Current gaps and challenges. Aliment. Pharmacol. Ther. 57, 1056–1065 (2023).
Siegel, R. C., Pinnell, S. R. & Martin, G. R. Cross-linking of collagen and elastin. Properties of lysyl oxidase. Biochemistry 9, 4486–4492 (1970).
Pinnell, S. R. & Martin, G. R. The cross-linking of collagen and elastin: Enzymatic conversion of lysine in peptide linkage to alpha-aminoadipic-delta-semialdehyde (allysine) by an extract from bone. Proc. Natl. Acad. Sci. 61, 708–716 (1968).
Radić, J. et al. Multiple roles of LOXL2 in the progression of hepatocellular carcinoma and its potential for therapeutic targeting. Int. J. Mol. Sci. 24, 11745 (2023).
Wong, C.C.-L. et al. Lysyl oxidase-like 2 is critical to tumor microenvironment and metastatic niche formation in hepatocellular carcinoma. Hepatol. Baltim. Md 60, 1645–1658 (2014).
Liburkin-Dan, T., Toledano, S. & Neufeld, G. Lysyl oxidase family enzymes and their role in tumor progression. Int. J. Mol. Sci. 23, 6249 (2022).
Moon, H.-J. et al. MCF-7 Cells expressing nuclear associated lysyl oxidase-like 2 (LOXL2) exhibit an epithelial-to-mesenchymal transition (EMT) phenotype and are highly invasive in vitro*. J. Biol. Chem. 288, 30000–30008 (2013).
Puente, A. et al. LOXL2—A new target in antifibrogenic therapy?. Int. J. Mol. Sci. 20, 1634 (2019).
Bignon, M. et al. Lysyl oxidase-like protein-2 regulates sprouting angiogenesis and type IV collagen assembly in the endothelial basement membrane. Blood 118, 3979–3989 (2011).
D’Ambrosio, R. et al. A morphometric and immunohistochemical study to assess the benefit of a sustained virological response in hepatitis C virus patients with cirrhosis. Hepatol. Baltim. Md 56, 532–543 (2012).
Poynard, T. et al. Slow regression of liver fibrosis presumed by repeated biomarkers after virological cure in patients with chronic hepatitis C. J. Hepatol. 59, 675–683 (2013).
Lu, M. et al. Serum biomarkers indicate long-term reduction in liver fibrosis in patients with sustained virological response to treatment for HCV infection. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 14, 1044-1055.e3 (2016).
Rockey, D. C. Fibrosis reversal after hepatitic C virus elimination. Curr. Opin. Gastroenterol. 35, 137–144 (2019).
Tahata, Y. et al. Improved liver function after sustained virologic response enhanced prognosis in hepatitis C with compensated advanced liver fibrosis. Dig. Dis. Sci. 68, 2115–2122 (2023).
Yoon, J.-H. et al. Prognosis of patients with chronic hepatitis C genotype 1b infection treated using daclatasvir/asunaprevir after sustained virologic response: A 6-year multicenter prospective observational study. Medicina (Mex) 59, 1436 (2023).
Krassenburg, L. A. P. et al. Clinical outcomes following DAA therapy in patients with HCV-related cirrhosis depend on disease severity. J. Hepatol. 74, 1053–1063 (2021).
Verna, E. C. et al. DAA therapy and long-term hepatic function in advanced/decompensated cirrhosis: Real-world experience from HCV-TARGET cohort. J. Hepatol. 73, 540–548 (2020).
Zangneh, H. F. et al. Cost effectiveness of hepatocellular carcinoma surveillance after a sustained virologic response to therapy in patients with hepatitis c virus infection and advanced fibrosis. Clin. Gastroenterol. Hepatol. 17, 1840-1849.e16 (2019).
Tanaka, Y. et al. HCC risk post-SVR with DAAs in East Asians: Findings from the REAL-C cohort. Hepatol. Int. 14, 1023–1033 (2020).
Oze, T. et al. Post-treatment levels of α-fetoprotein predict incidence of hepatocellular carcinoma after interferon therapy. Clin. Gastroenterol. Hepatol. 12, 1186–1195 (2014).
Tahata, Y. et al. Risk of hepatocellular carcinoma after sustained virologic response in hepatitis C virus patients without advanced liver fibrosis. Hepatol. Res. 52, 824–832 (2022).
Yasui, Y. et al. Wisteria floribunda agglutinin-positive Mac-2 binding protein predicts early occurrence of hepatocellular carcinoma after sustained virologic response by direct-acting antivirals for hepatitis C virus: Predictive value of WFA + -M2BP after SVR. Hepatol. Res. 48, 1131–1139 (2018).
Tada, T. et al. Post-treatment levels of α-fetoprotein predict long-term hepatocellular carcinoma development after sustained virological response in patients with hepatitis C: AFP predicts HCC development in SVR patients. Hepatol. Res. 47, 1021–1031 (2017).
Sato, S. et al. Prediction of hepatocellular carcinoma development after hepatitis C virus eradication using serum wisteria floribunda agglutinin-positive Mac-2-binding protein. Int. J. Mol. Sci. 17, 2143 (2016).
Akuta, N. et al. Complex association of virus- and host-related factors with hepatocellular carcinoma rate following hepatitis C virus clearance. J. Clin. Microbiol. 57, e01463-e1518 (2019).
Kondili, L. A. et al. Profiling the risk of hepatocellular carcinoma after long-term HCV eradication in patients with liver cirrhosis in the PITER cohort. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 55, 907–917 (2023).
Watanabe, T. et al. Simple new clinical score to predict hepatocellular carcinoma after sustained viral response with direct-acting antivirals. Sci. Rep. 13, 8992 (2023).
Hiraoka, A. et al. Validation and potential of albumin-bilirubin grade and prognostication in a nationwide survey of 46,681 hepatocellular carcinoma patients in Japan: The need for a more detailed evaluation of hepatic function. Liver Cancer 6, 325–336 (2017).
Hiraoka, A. et al. Validation of modified ALBI grade for more detailed assessment of hepatic function in hepatocellular carcinoma patients: A multicenter analysis. Liver Cancer 8, 121–129 (2019).
Imai, K. et al. FIB-4 index and NAFLD fibrosis score are useful indicators for screening high-risk groups of non-viral hepatocellular carcinoma. Mol. Clin. Oncol. 19, 80 (2023).
Sato, S. et al. Post-treatment serum Wisteria floribunda agglutinin-positive mac-2-binding protein level is a useful predictor of hepatocellular carcinoma development after hepatitis C virus eradication. JGH Open 5, 1203–1209 (2021).
Cano, A., Eraso, P., Mazón, M. J. & Portillo, F. LOXL2 in cancer: A two-decade perspective. Int. J. Mol. Sci. 24, 14405 (2023).
Matsuo, A. et al. Significance of nuclear LOXL2 inhibition in fibroblasts and myofibroblasts in the fibrotic process of acute respiratory distress syndrome. Eur. J. Pharmacol. 892, 173754 (2021).
Wen, B., Xu, L.-Y. & Li, E.-M. LOXL2 in cancer: Regulation, downstream effectors and novel roles. Biochim. Biophys. Acta BBA Rev. Cancer 1874, 188435 (2020).
Cuevas, E. P. et al. LOXL2 catalytically inactive mutants mediate epithelial-to-mesenchymal transition. Biol. Open 3, 129–137 (2014).
Ružić, M. et al. Hepatitis C virus-induced hepatocellular carcinoma: A narrative review. Panminerva Med. 60, 185–191 (2018).
Dash, S., Aydin, Y., Widmer, K. E. & Nayak, L. Hepatocellular carcinoma mechanisms associated with chronic HCV infection and the impact of direct-acting antiviral treatment. J. Hepatocell. Carcinoma 7, 45–76 (2020).
Qiu, L., Xu, S., Qiu, Y., Liu, Y. & Zhang, J. Comparison of acknowledged hepatocellular carcinoma risk scores in high-risk hepatitis C patients with sustained virological response. J. Viral Hepat. 30, 559–566 (2023).
Kawaguchi, T. et al. Enhanced liver fibrosis score as a predictive marker for hepatocellular carcinoma development after hepatitis C virus eradication. Mol. Clin. Oncol. 15, 215 (2021).
Caviglia, G. P. et al. Long-term hepatocellular carcinoma development and predictive ability of non-invasive scoring systems in patients with HCV-related cirrhosis treated with direct-acting antivirals. Cancers 14, 828 (2022).
Rocha, C. et al. Hepatocellular carcinoma in patients cured of chronic hepatitis C: Minimal steatosis. Cancer Med. 12, 10175–10186 (2023).
Harrison, S. A. et al. Simtuzumab is ineffective for patients with bridging fibrosis or compensated cirrhosis caused by nonalcoholic steatohepatitis. Gastroenterology 155, 1140–1153 (2018).
Chen, W. et al. Lysyl oxidase (LOX) family members: Rationale and their potential as therapeutic targets for liver fibrosis. Hepatol. Baltim. Md 72, 729–741 (2020).
Gong, L. et al. Inhibition of lysyl oxidase-like 2 overcomes adhesion-dependent drug resistance in the collagen-enriched liver cancer microenvironment. Hepatol. Commun. 6, 3194–3211 (2022).
Klepfish, M. et al. LOXL2 inhibition paves the way for macrophage-mediated collagen degradation in liver fibrosis. Front. Immunol. 11, 480 (2020).
Tsai, P.-C. et al. Metformin reduces hepatocellular carcinoma incidence after successful antiviral therapy in patients with diabetes and chronic hepatitis C in Taiwan. J. Hepatol. 78, 281–292 (2023).
Ciancio, A. et al. Long-term follow-up of diabetic and non-diabetic patients with chronic hepatitis C successfully treated with direct-acting antiviral agents. Liver Int Off. J. Int. Assoc. Study Liver 41, 276–287 (2021).
Hedenstierna, M., Nangarhari, A., Weiland, O. & Aleman, S. Diabetes and cirrhosis are risk factors for hepatocellular carcinoma after successful treatment of chronic hepatitis C. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 63, 723–729 (2016).
Lockart, I., Yeo, M. G. H., Hajarizadeh, B., Dore, G. J. & Danta, M. HCC incidence after hepatitis C cure among patients with advanced fibrosis or cirrhosis: A meta-analysis. Hepatol. Baltim. Md 76, 139–154 (2022).
Johnson, P. J. et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 33, 550–558 (2015).
Vallet-Pichard, A. et al. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection comparison with liver biopsy and fibrotest. Hepatol. Baltim. Md. 46, 32–36 (2007).
Acknowledgements
We thank Y. Kobayashi, M.D., Ph.D., for conceptualization. We would like to thank Editage (www.editage.com) for the English language editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization: T.C., K.K. Sample collection: T.C., K.O., H.N., K.K., G.M., F.K., Y.S., M.M., T.O., T.N., K.T., M.T. Methodology: T.C., K.O. Investigation: T.C. Supervision: K.K., T.S. Writing—original draft: T.C. Writing—review & editing: T.C., K.K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chida, T., Ohta, K., Noritake, H. et al. Lysyl oxidase-like 2 as a predictor of hepatocellular carcinoma in patients with hepatitis C virus after sustained virological response. Sci Rep 14, 10864 (2024). https://doi.org/10.1038/s41598-024-61366-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-61366-y
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.