Abstract
Recently it has been recognized that herbal plants contain endogenous molecules with biostimulant properties, capable of inducing morphological and biochemical changes in crop plants. Therefore, the present experiment was conducted to screen herbal samples for their plant growth promoting properties. Twenty-five herbal extracts were tested for their biostimulating activity on wheat crop (Triticum aestivum) through seed priming. Morphological parameters chosen for evaluation include: percent seed germination, length and weight of seedling, wheat grass length and biomass. Biochemical parameters include: total phenolic and flavonoid, enzymatic activity of catalase and phenylalanine ammonium lyase and antioxidant activity. Results indicated an increase in the tested parameters by the extracts, however the biostimulant property varied between the selected herbal samples. Some of the samples, such as Phyllanthus emblica, Plumbago zeylanica, Catharanthus roseus and Baccopa monnieri, were highly effective in inducing plant growth promoting parameters. Principal component analysis was performed and herbal samples were grouped into categories based on their activity.
Similar content being viewed by others
Introduction
It is indisputable that wheat is an economically significant crop contributing to both human and animal diets. It is a significant source of calories and also contain a variety of nutrients, including phytochemicals, vitamins and dietary fibres that are required for maintaining a healthy body1. Along with wheat grains, wheatgrass has long been a staple of Indian culture and is renowned for its exceptional medicinal qualities. It is a vegetable that is picked before the plant blooms2. Regular consumption of wheatgrass extract enhances digestion and improves overall health3,4,5.
Priming is the term for seed energising procedures carried out before germination6. Seed priming increases germination speed and uniformity while also causing several biochemical changes that are essential for breaking dormancy, mobilising or hydrolysing seed stores, activating enzymes and promoting plant growth and development7,8. To enhance seed germination and plant development, commercial growth regulators have been utilised as priming agents. Exogenous biostimulants have successfully benefited crop plants, such as fruits, vegetables cereals and legumes for better growth, phytochemical, crop yield and quality9,10,11.
It has been reported that some plant compounds influence crop growth and yield. Seed priming with sunflower (Helianthus annuus L.)12, moringa (Moringa oleifera Lam.)13 and sorghum (Sorghum bicolor L.)14 leaf extracts enhanced the emergence rate, seedling growth, and development of maize (Zea mays L.) and wheat (Triticum aestivum L.) crop. Zeatin and other plant growth boosters were found in abundance in the extract of moringa leaves15, whereas phenolic acids, such as vanillic, coumaric, ferulic and hydroxybenzoic acids were identified in sorghum extract as biostimulating agents16. Exogenous application of aqueous garlic extracts on eggplant and pepper seedlings improved growth and phytochemical content of the treated plants17. Leaf extracts from aloe (Aloe barbadensis L.) and heena (Lawsonia inermis L.) improved the growth and chemical composition of Schefflera arboricola Hayata (umbrella plant)18. Oak bark extract improved colour, polyphenols contents, as well as aroma of grapevines. Glycyrrhiza glabra L. (Licorice) root extract has been shown to improve growth and yield of beans. Because of the widespread applicability of natural extracts in modulating plant growth and development, the present study was set up to assess the potential effects of some herbal extracts on the germination process as well as their contribution to the accumulation of bioactive compounds in wheatgrass.
Materials and methods
Experimental setup
All methods used in the work were carried out in accordance with relevant guidelines25. The studies were conducted in the laboratory of Banaras Hindu University (BHU) Varanasi. Wheat was cultivated in a plant growth chamber with constant humidity (70%) and temperature (25 °C ± 2 °C), while maintaining 16 h of light and 8 h of darkness throughout the study.
Herbal extract preparation
Herbal extracts were obtained from twenty-five species of herbal plants (Table 1). Stock solutions were prepared using plant tissues, primarily leaves, by homogenising 0.2 g of the fresh tissue in 15 ml of distilled water with the help of pestle and mortar. The extract obtained was immediately filtered through Whatmann filter paper and different dilutions were prepared by taking 2 ml (Solution A), 4 ml (Solution B), and 8 ml (Solution C) of stock solution and achieving a final volume of 20 ml by adding distilled water.
Seed collection
HVW-243 wheat (Triticum aestivum L.) variety, one of the oldest and widely cultivated variety in the eastern Gangetic plains, and containing various nutritional composition was selected as experimental material. HVW-243 was purchased from agricultural farm of Banaras Hindu University Varanasi, Uttar Pradesh.
Germination test
Seeds were surface sterilised with 2% sodium hypochlorite solution, followed by a thorough rinse with distilled water. Then, seeds were soaked for 8 h at room temperature in different herbal extract concentrations; distilled water serving as the control. Ten seeds were spread on a Petri dish containing double layers of moist filter paper. Petri dishes were kept in complete darkness at room temperature for germination. After germination, seedling morphological parameters were calculated, including seedling length, biomass and percentage germination, according to the following formula.
with GP representing the germination percentage, GS the number of germinated seed, and TS the number of sown seeds. For each germination condition, five replicates of each treatment were performed.
Pot experiment
Seeds were soaked in herbal extracts as well as in distilled water (control), as described above. Thirty seeds were placed in each pot, containing sterilized soil, and were then allowed to grow for 10 days in a plant growth chamber (conditions described above). Plants were watered with sterile distilled water at an interval of three days. After ten days, plants were harvested and wheatgrass length and fresh weight were calculated.
Estimation of total phenolic content
The Folin–Ciocalteau method was used to measure the total phenolic content, according to the methods described in the literature19. One gram of dry biomass of wheat leaves was taken and extracted with 10 ml of 70% methanol. A test tube containing about 0.5 ml of 70% methanolic extract and 0.5 ml of methanol were taken and 300 µl of 50% diluted Folin–Ciocalteau's phenol reagent was added. The reaction mixture was left at room temperature for 10 min in the dark, after which twenty percent aqueous sodium carbonate solution was added. Distilled water was then added to keep the sample's final volume at 3 ml. At 650 nm, the absorbance of the reaction mixture was measured after standing for 60 min at room temperature. As a control, a standard curve was created using gallic acid at various concentrations.
Estimation of total flavonoid
The total flavonoid content of wheatgrass was measured using reported protocol20. One ml of seventy percent of methanolic extract was combined with 1 ml of pure methanol and then 1 ml of 2% AlCl3 was added. This combination was incubated for 1 h at room temperature, after which absorbance was measured at 420 nm. In order to prepare a conventional graph, Rutin was utilised as standard.
Photosynthetic pigments
To estimate the photosynthetic pigments, the method of Takshak and Agrawal 201821 was applied with slight modification. Wheatgrass powder was homogenised in acetone and centrifuged at 5000 rpm for five minutes to extract photosynthetic pigments. Supernatant was collected and the absorbance was measured at 480, 510, 645, and 663 nm to estimate the presence of carotenoids, chlorophyll b (Chl b) and chlorophyll a (Chl a) using the following formulas:
V is the volume of the extract (ml) and W is the fresh weight of the sample (g).
2,2-Diphenyl-1-picrythydrazyl radical assay (DPPH)
The wheatgrass samples were homogenised in 70% methanol and then centrifuged at 5000 rpm for five minutes. The supernatant was collected and used for the measurement of antioxidant activity by DPPH radical scavenging assay22,23. In a test tube, 0.5 ml of extract was taken and diluted with 2.5 ml of methanol. The sample mixture was combined with 1 ml of 0.004% DPPH solution and incubated for 30 min in the dark. Ascorbic acid was used as standard. The absorbance was measured at 517 nm and the proportion of antioxidant activity was determined using the following formula:
AC representing absorbance of control and AS representing absorbance of sample. Methanolic wheatgrass extract was the sample and 3 ml methanol were used as control.
Ferric- reducing antioxidant power assay (FRAP)
The FRAP assay was carried out using Guo methodology24. Approximately 0.5 ml of extract was taken and diluted with 0.5 ml of methanol, followed by the addition of 2 ml of FRAP reagent. It was incubated for 15 min at 37 °C, after which absorbance was calculated at 593 nm. By comparing antioxidant activity to ascorbic acid standard solutions, antioxidant activity of the extract was estimated. Fresh FRAP reagent was prepared by combining 2.5 ml of 10 mmol/l 2,4,6-Tripyridy-s-triazine (TPTZ) produced in 40 mmol/l HCl with 2.5 ml of 20 mmol/l FeCl3 (prepared in 25 ml of 0.3 mol/L acetate buffer, pH 3.6).
Peroxidase (POD) activity
Using a chilled mortar and pestle, 2 g of fresh wheatgrass was homogenised in 2 ml of 0.1 M cold phosphate buffer (7 pH). Supernatant of sample was taken and enzyme activity was calculated using method described in literature25. A cuvette was filled with 3 ml of 0.1 M phosphate buffer (pH 7.0), 0.05 ml of 4% guaiacol and 0.1 ml of extract and was thoroughly mixed. Spectrophotometer was set to 436 nm, the cuvette was placed and 0.03 ml of 30% H2O2 was added. Time taken to raise the absorbance by 0.1 was noted. The control was phosphate buffer with guaiacol solution.
Phenylalanine ammonium-lyase (PAL) activity
The quantity of cinnamic acid produced by the enzyme's reaction was used to assess the activity of PAL enzyme26. The reaction mixture contained 0.5 ml of crude extract along with 1 ml of 10 mM Phenylalanine, prepared in 0.1 M Tris–HCl buffer of pH 8.5. After being allowed to continue for 30 min at room temperature, the reaction was halted by adding 0.5 ml of 2 N HCl. The absorbance was measured at 290 nm and a typical graph showing the relationship between absorbance and cinnamic acid content was created. The nmole of cinnamic acid generated during the deamination of l-phenylalanine under normal conditions is comparable to one unit of PAL activity.
Statistical analysis
Each herbal extract treatment had five repetitions, 10 seeds were taken for the germination test and 30 seeds were taken for the pot experiment for each replicate. All tests were carried out twice. The means and standard error (SE) are displayed as error bars on the graph. The effects of the herbal extract on the biochemical, morphological and yield features of wheatgrass were analysed using a one-way univariate analysis of variance (ANOVA). Following the one-way ANOVA, Duncan's multiple range tests were run as post hoc analyses for various parameters. The SPSS statistical package (SPSS Inc., version 16.0) was used for all statistical calculations. PCA analysis was performed by using regression analysis, varimax rotation, and correlation matrix. IBM SPSS (SPSS Inc., version 16.0), Sigmaplot, and Minitab were used for statistical analyses and graph preparation.
Result and discussion
Twenty-five herbal samples with three dilutions of each extract were tested for their plant growth promoting activity. Effect of the treatments on morphological and biochemical parameters of wheat sprout and wheatgrass is shown in Tables 2 and 3. Data analysis revealed an increase in the tested parameters by the treatments, however, the effect varied with the type of herbal extract and its dilution. Figures 1 and 2 displays a comparative assessment of biostimulant activity of all the 25 herbal samples, taking best dilution of each extract for graph preparation. A detailed analysis of the experimental result is presented below.
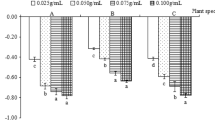
Effect of different herbal extract treatment on morphological parameters of wheat sprouts and wheatgrass (A) Seedling length, (B) Seedling weight (C) Wheatgrass length (D) Wheatgrass weight. Values are the means ± standard deviation at significance difference p < 0.05 (different superscripts, a, b, c, d e, bc, cd shows significantly different values).
Effect of different herbal extract treatment on biochemical parameters of wheatgrass. (A) DPPH assay, (B) FRAP assay, (C) Total phenolic, (D) Total flavonoids, (E) POD activity, (F) PAL activity, (G) Total chlorophyll and (H) Total carotenoid. Data are representative of five replicates. Values are the means ± standard deviation, at significance difference p < 0.05 (different superscripts, a, b, c, d e, bc, cd shows significantly different values).
Effect of herbal extracts on wheat seedling: germination percentage, seedling length, and seedling weight
The process of germination starts by the quiescent dry seeds absorbing water and ending with the lengthening of the embryonic axis27. Our study revealed that all herbal extract treatment enhanced the germination rate, germination percentage, seedling length and seedling weight of wheat sprouts (Table 2). Lower concentrations (solution A and B) of all treatment showed 100% germination (similar to control), however higher concentrations (solution C) of some herbal extracts showed inhibitory character, demonstrating lower germination rate (Table 2). Seedling length and weight also increased with herbal extract treatment; however higher concentrations of some extracts were found to decrease length. The solution C of Phyllanthus emblica L. showed maximum increase in seedling length and seedling weight, followed by solution B of Plumbago zeylanica L. and solution A of Bacopa monnieri L., with 2.33-fold, 2.20-fold and 2.0-fold increase in length and 5.0-fold, 4.57-fold and 3.91-fold increase in seedling weight respectively as compared to control (Fig. 1A,B). It may be concluded that the rate of metabolism was accelerated by seed priming, hastening germination and emergence of seedling. Panuccioa et al.28 discovered that Zea mays L. (maize) seeds treated with Rosmarinus officinalis or Artemisia leaf extracts had higher germination percentage. Similarly, priming tomato seeds with Vinca, Chlorophytum and Azadirachta has been shown to reduce mortality and increase seedling vigour29. Herbal plants contain bioactive compounds, such as phytohormones, growth regulators, phenolic acid30, which might have played a significant role in regulating the metabolism associated with seed germination and seedling establishment. Our research showed that while a particular concentration of herbal extract stimulated growth, excess of this dose reduced it. Literature reports have documented a negative effect of higher concentration of biostimulant on germination rate23,25.
Effect of treatment on wheatgrass length and fresh biomass
Wheatgrass is a term used to describe wheat germinated during a period of 6–10 days. Wheatgrass has recently gained interest as a health food in various countries and consumed in the form of a ready-made beverage or pill31. Wheatgrass is thought to be a good source of vitamins, antioxidants, and minerals32. We observed in our experiment that after seed treatment, there was an increase in plant biomass due to enhanced growth and increased height, however, reduction was seen in many of the highly concentrated herbal extract treatment (Table 2). Among the tested 25 extract, highest gain in wheatgrass length and biomass were found in P. emblica (1.29-fold and 2.08-fold) followed by P. zeylanica (1.28-fold and 2-fold) and B. monnieri (1.20-fold and 1.45-fold) than control, whereas Rauvolfia serpentina L., Withania somnifera L. and Syzygium aromaticum L. were least effective treatments (Fig. 1C,D). Some plant-based extracts have recorded improved growth and biomass accumulation in crops after their treatment; such as, moringa leaf extract improved wheat growth and productivity15, foliar spraying with seaweed and licorice root extracts improved the vegetative growth of onion seedlings33. Similarly, the application of Alfalfa derived protein hydrolysates improved tomato biomass and quality34.
Effect of treatment on antioxidant activity
Through a process known as oxidative stress, environment has a harmful effect on the body35. Numerous diseases, including cancer, arthritis, ageing, autoimmune disease, cataract, cardiovascular and neurological disorders are greatly influenced by oxidative stress36,37,38. The presence of substances that can shield a biological system from the detrimental effects of oxidative stress is connected to the antioxidant capability. The increase of protective molecules in the form of antioxidant compounds was another finding from our study. The ability of DPPH to scavenge free radicals and the ability of the FRAP reagent to reduce them were used to assess the antioxidant potential of wheatgrass. In comparison to the control, each treatment considerably increased the antioxidant potential; either through a higher or a lower concentration dose (Table 3). Moderately concentrated leaf extract of P. emblica (Solution B) was effective and showed the maximum increase by FRAP (1.94-fold) and DPPH (3.11-fold) method, followed by leaf extract of P. zeylanica and B. monnieri (Solution C) causing a rise of 1.93-fold and 1.82-fold by FRAP and 2.28-fold and 2.38-fold by DPPH method, respectively (Fig. 2A,B). Exogenous application of biostimulant has reportedly been shown to activate both enzymatic and non-enzymatic antioxidant defence mechanisms in plants25,26. Dawoud39 discovered a rise in DPPH and FRAP levels and came to the conclusion that Punica granatum L. peel extract contains biostimulants that could improve health-promoting metabolites in B. oleraceae sprouts. The antioxidant and health-promoting qualities of kidney bean sprouts have also been enhanced by exogenous treatment with natural elicitors40. In Solanum melongena L., Ali et al.41 showed that the antioxidant system can be modified in response to the priming effect of an aqueous garlic extract.
Effect of treatment on phenolic content
Due to its high antioxidant activity, wheatgrass juice is renowned as a healthful beverage. Ferulic acid, caffeic acid, p-coumaric acid, gallic acid, and syringic acid are the principal phenolic chemicals present in wheatgrass42. The flavonoid apigenin, which has potential anti-carcinogenic and anti-oxidant properties, is notably abundant in wheatgrass43. By doing this investigation, we were able to increase the total phenolic and flavonoid contents of wheatgrass. Some of the tested extracts displayed multifold increase while others caused mild changes in phenolic levels (Table 3). P. emblica extracts exhibited 2.32-fold phenol and 2.43-fold flavonoid, P. zeylanica showed 2.26-fold phenol and 2.38-fold flavonoids and B. monnieri manifested 2.02-fold phenol and 0.88-fold flavonoid increase while extracts of R. serpentina W. somnifera and S. aromaticum were least effective (Fig. 2C,D). According to previously published study, priming with biostimulants have improved the levels of phenols, flavonoids, and antioxidant systems in wheat and rice crops25,44,45. Using leaf extracts from Typha angustifolia, Ghezal et. al.46 discovered that the secondary metabolites, including total phenolic and total flavonoid in pea (Pisum sativum L.) was increased.
Effect of treatment on enzyme activity
An essential antioxidant enzyme that protects plant against oxidative stress is peroxidases. They catalyze detoxification of hydrogen peroxide and convert them into water22. Increase in POD activity by exogenous application of chemical biostimulant had been observed in several studies23,26. Similarly, in many plant species, the induction of PAL enzyme activity by chemical elicitors is associated with the build-up of phenolic chemicals47. Therefore, an experiment was created to investigate the induction of POD and PAL activity by herbal extracts. An increase in enzyme activity was observed in wheatgrass after treatment, although variation in effect was reflected within treatments (Table 3) The extracts from P.emblica, P. zeylanica, and B. monnieri were the ones with the highest POD activity (4.11-fold, 3.93-fold and 3.64-fold respectively) while extracts of R. serpentina (1.25-fold), W. somnifera (1.29-fold), and S. aromaticum (1.33 fold) were the least effective one (Fig. 2E). Similarly, the highest PAL activities were shown by the extracts from B. monnieri, P. zeylanica, and P. emblica, which were 5.35-fold, 4.34-fold, and 3.53-fold, respectively and the lowest PAL activities were shown by the extracts from R. serpentina, W. somnifera, and S. aromaticum, which were 1.49-fold, 1.49-fold, and 1.52-fold respectively (Fig. 2F).
Effect of treatment on photosynthetic pigments
Chlorophyll has long been recognised as a source of energy for plant metabolism. The chlorophyll concentration of the cell influences photosynthetic effectiveness43. In literature reports, the benefits of wheatgrass juice have been linked to its high chlorophyll concentration. Animal models used in scientific research on the health benefits of chlorophyll have demonstrated anti-cancer effects48. According to earlier studies, seed priming with a variety of naturally occurring chemicals aided in the enhancement of photosynthetic pigments. An increase in the anthocyanin and chlorophyll content in seeds primed with biostimulants was reported in rice seedlings49. The priming of mung bean plant with natural compounds helped them to cope with a number of abiotic challenges, by improving the photosynthesis process and increasing the photosynthetic pigments50. However, in our experiment it was observed that herbal extract treatments were less efficient in modulating the total amount of chlorophyll and carotenoids as compared with other tested parameters. The majority of treatments were found to have a non-significant difference in pigment content as compared with the control (Table 3). Pigment level was inversely connected with treatments of highly concentrated herbal extracts and correlated favourably with treatments of lesser concentration. The increase in total chlorophyll was highest in P. emblica (2.05-fold), P. zeylanica (1.99-fold), and B. monnieri (1.97-fold) (Fig. 2G). Carotenoid concentration was also found to be positively connected with treatments using less concentrated herbal extracts and adversely correlated with highly concentration treatments (Table 3). Like chlorophyll, a small number of therapies exhibited significant differences, with highest carotenoid percentage rise observed in P. emblica (0.66-fold), P. zeylanica (0.64-fold) and B. monnieri (0.61-fold) treatments (Fig. 2H).
Principal Component Analysis (PCA)
Finding patterns and correlations in data is the major objective of a PCA analysis51. The first two primary components were used to produce the score plot, and accounted for 97.4% of the total variance. PCA provided us with a macroscopic categorization that allowed us to separate the herbal samples according to how well they may cause alterations. From the score plot, we could find that all the 25 samples can be clustered into four groups (Fig. 3). Out of these groups, the purple circle, comprising P. emblica, P. zeylanica, C. roseus, and B. monnieri have the greatest potential to stimulate secondary metabolite production in wheatgrass, followed by C. roseus, O. tenuiflorum, A. indica, and T. erecta, which are circled in yellow. Green circle indicates that A. physocarpa, E. prostrata, P. longum, N. arbortristis, C. camphora, P. lanceolate, C. citratus, M. piperita, and F. religiosa are moderately active to boost secondary metabolites synthesis in wheatgrass and the blue circle indicates that the extract of H. isora, C. indicum, A. racemosus, J. Grandiflorum, T. cordifolia, S. aromaticum, W. somnifera, and R. serpentine are least effective in inducing changes (Fig. 3).
Results of the PCA analysis clearly shows a variation in biostimulating activity between herbal samples. Next, we were interested to quantify the effectiveness of the extracts based on their activity. Therefore, we divided these 25 herbal samples into three groups as most effective, moderate effective and least effective (each group comprising around eight samples) and calculated their overall contribution in enhancing the growth characteristics. The most effective herbal extract is shown in the blue area of the pie chart and covers around 47–68% of morphological parameters (Fig. 4) and 47–56% of biochemical parameters (Fig. 5). The moderately effective herbal extract percentage is depicted in the orange area of the pie chart and accounts for 23–36% of tested parameters, whereas the least effective herbal extract percentage (grey area) ranges between 9 and 27% (Figs. 4 and 5). The result shows a variation in the activity of the tested herbal samples; where some of the extracts are causing multi-fold increase in growth parameters, while some are completely ineffective. This variation depends on the chemical composition of the extracts and the concentration of their bioactive compounds.
Conclusion
Natural compounds in agriculture are increasingly popular due to the environmental impact of synthetic chemicals and rising demand for organic foods. This study highlights the potential of various herbal extracts as natural biostimulants, showing their positive effects on wheat germination, biomass, and antioxidant activities. The effectiveness of the herbal extracts varies with the plant type and concentration, indicating a need for future research to identify and characterize these bioactive compounds for wider agricultural applications. This aligns with the current trend towards sustainable agriculture, where natural growth stimulants play a crucial role.
Data availability
All data generated or analysed during this study are included in this published article.
References
Shewry, P. R. & Hey, S. J. The contribution of wheat to human diet and health. Food Energy Secur. 4, 178–202. https://doi.org/10.1002/fes3.64 (2015).
Rana, S., Kamboj, J. K. & Gandhi, V. Living life the natural way–Wheatgrass and Health. Funct. Foods Health Dis. 11, 444–456. https://doi.org/10.31989/ffhd.v1i11.112 (2011).
Ben-Arye, E. et al. Wheat grass juice in the treatment of active distal ulcerative colitis: A randomized double-blind placebo-controlled trial. Scand. J. Gastroenterol 37, 444–449. https://doi.org/10.1080/003655202317316088 (2002).
de Vogel, J., Jonker-Termont, D. S., Katan, M. B. & van der Meer, R. Natural chlorophyll but not chlorophyllin prevents heme-induced cytotoxic and hyperproliferative effects in rat colon. J. Nutr. 135, 1995–2000. https://doi.org/10.1093/jn/135.8.1995 (2005).
Ferruzzi, M. G. & Blakeslee, J. Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutr. Res 27, 1–12. https://doi.org/10.1016/j.nutres.2006.12.003 (2007).
Bradford, K. J. Manipulation of seed water relations via osmotic priming to improve germination under stress conditions. Horm. Res. 21, 1105–1112. https://doi.org/10.21273/HORTSCI.21.5.1105 (1986).
Schreiner, M. & Huyskens-Keil, S. Phytochemicals in fruit and vegetables: Health promotion and postharvest elicitors. CRC Crit. Rev. Plant Sci. 25, 267–278. https://doi.org/10.1080/07352680600671661 (2006).
Catav, S. S., Bekar, I., Ates, B. S., Ergan, G. & Oymak, F. Germination response of five eastern Mediterranean woody species to smoke solutions derived from various plants. Turk. J. Bot. 36, 480–487. https://doi.org/10.3906/bot-1111-12 (2012).
Singh, S. Role of nonpathogenic fungi in inducing systemic resistance in crop plants against phytopathogens. in Microbial Inoculants in Sustainable Agricultural Productivity, Vol. 2 (2016).
Park, C. H. et al. Influence of indole-3-acetic acid and gibberellic acid on phenylpropanoid accumulation in common buckwheat (Fagopyrum esculentum Moench) sprouts. Molecules 22, 374. https://doi.org/10.3390/molecules22030374 (2017).
Zhao, T. et al. IAA priming improves the germination and seedling growth in cotton (Gossypium hirsutum L.) via regulating the endogenous phytohormones and enhancing the sucrose metabolism. Ind. Crops Prod. 155, 1–8. https://doi.org/10.1016/j.indcrop.2020.112788 (2020).
Baldwin, I. T., Kessler, A. & Halitschke, R. Volatile signaling in plant–plant–herbivore interactions: What is real?. Curr. Opin. Plant Biol. 5, 351–354. https://doi.org/10.1016/S1369-5266(02)00263-7 (2002).
ur Rehman, H. et al. Seed priming improves early seedling vigor, growth and productivity of spring maize. J. Integr. Agric. 14, 1745–1754. https://doi.org/10.1016/S2095-3119(14)61000-5 (2015).
Bajwa, A. A. & Farooq, M. Seed priming with sorghum water extract and benzyl amino purine along with surfactant improves germination metabolism and early seedling growth of wheat. Arch. Agron. Soil Sci. 63, 319–329. https://doi.org/10.1080/03650340.2016.1211268 (2017).
Rehman, H. U., Basra, S., Rady, M. M., Ghoneim, A. M. & Wang, Q. Moringa leaf extract improves wheat growth and productivity by affecting senescence and source-sink relationship. Int. J. Agric. Biol. 19, 479–484. https://doi.org/10.17957/IJAB/15.0316 (2017).
Basra, S. M. A., Iftikhar, M. N. & Afzal, I. Potential of moringa (Moringa oleifera) leaf extract as priming agent for hybrid maize seeds. Int. J. Agric. Biol. 13, 1006–1010 (2011).
Hayat, S. et al. Aqueous garlic extract as a plant biostimulant enhances physiology, improves crop quality and metabolite abundance, and primes the defense responses of receiver plants. Appl. Sci. 8, 1505 (2018).
Zulfiqar, F., Casadesús, A., Brockman, H. & Munné-Bosch, S. An overview of plant-based natural biostimulants for sustainable horticulture with a particular focus on moringa leaf extracts. Plant Sci. 295, 110194. https://doi.org/10.1016/j.plantsci.2019.110194 (2020).
Chavan, J. J., Gaikwad, N. B., Kshirsagar, P. R. & Dixit, G. B. Total phenolics, flavonoids and antioxidant properties of three Ceropegia species from Western Ghats of India. S. Afr. J. Bot. 88, 273–277. https://doi.org/10.1016/j.sajb.2013.08.007 (2013).
Ordonez, A. A. L., Gomez, J. D. & Vattuone, M. A. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 97, 452–458. https://doi.org/10.1016/j.foodchem.2005.05.024 (2006).
Takshak, S. & Agrawal, S. B. Interactive effects of supplemental ultraviolet-B radiation and indole-3-acetic acid on Coleus forskohlii Briq. Alterations in morphological-, physiological-, and biochemical characteristics and essential oil content. Ecotoxicol. Environ. Saf. 147, 313–326. https://doi.org/10.1016/j.ecoenv.2017.08.059 (2018).
Chen, Z. et al. Comparison of phenolic profiles, antioxidant capacity and relevant enzyme activity of different Chinese wheat varieties during germination. Food Biosci. 20, 159–167. https://doi.org/10.1016/j.fbio.2017.10.004 (2017).
Singh, S. Salicylic acid elicitation improves antioxidant activity of spinach leaves by increasing phenolic content and enzyme levels. Food Chem. Adv. 2, 1–11. https://doi.org/10.1016/j.focha.2022.100156 (2023).
Guo, C. et al. Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nutr. Res. 23, 1719–1726. https://doi.org/10.1016/j.nutres.2003.08.005 (2003).
Yadav, A. & Singh, S. Effect of exogenous phytohormone treatment on antioxidant activity, enzyme activity and phenolic content in wheat sprouts and identification of metabolites of control and treated samples by UHPLC-MS analysis. Food Res. Int. 169, 112811. https://doi.org/10.1016/j.foodres.2023.112811 (2023).
Singh, S. Enhancing phytochemical levels, enzymatic and antioxidant activity of spinach leaves by chitosan treatment and an insight into the metabolic pathway using DART-MS technique. Food Chem. 199, 176–184. https://doi.org/10.1016/j.foodchem.2015.11.127 (2016).
Rath, D., Dash, A. K. & Padhi, S. Effect of low frequency (50 Hz) electromagnetic field on germination process of wheat (Triticum aestivum L.). Sch. Acad. J. Biosci. 11, 147–158. https://doi.org/10.3390/agronomy13020454 (2023).
Panuccio, M. R., Chaabani, S., Roula, R. & Muscolo, A. Bio-priming mitigates detrimental effects of salinity on maize improving antioxidant defense and preserving photosynthetic efficiency. Plant Physiol. Biochem. 132, 465–474. https://doi.org/10.1016/j.plaphy.2018.09.033 (2018).
Prabha, D., Negi, S., Kumari, P., Negi, Y. K. & Chauhan, J. S. Effect of seed priming with some plant leaf extract on seedling growth characteristics and root rot disease in tomato. Int. J. Agric. Syst. 4, 46–51. https://doi.org/10.20956/ijas.v4i1.240 (2016).
Fuglie LJ. The miracle tree: Moringa oleifera. Natural Nutrition for the Tropics. pp. 68. CWS, Dakar, Senegal (1999).
Kulkarni, S. D. et al. Evaluation of the antioxidant activity of wheatgrass (Triticum aestivum L.) as a function of growth under different conditions. Phytother. Res. 20, 218–227. https://doi.org/10.1002/ptr.1838 (2006).
Chauhan, M. A pilot study on wheat grass juice for its phytochemical, nutritional and therapeutic potential on chronic diseases. Int. J. Chem. Stud. 2, 27–34 (2014).
Babilie, R., Jbour, M. & Trabi, B. A. Effect of foliar spraying with licorice root and seaweed extractson growth and seed production of onion (Allium cepa L.). Int. J. Chemtech. Res. 8, 557–563 (2015).
Ertani, A., Schiavon, M. & Nardi, S. Transcriptome-wide identification of differentially expressed genes in Solanum lycopersicon L. in response to an alfalfa-protein hydrolysate using microarrays. Front. Plant Sci. 8, 1–19. https://doi.org/10.3389/fpls.2017.01159 (2017).
Valko, M., Rhodes, C. J. B., Moncol, J., Izakovic, M. M. & Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Interact 160, 1–40. https://doi.org/10.1016/j.cbi.2005.12.009 (2006).
Birben, E., Sahiner, U. M., Sackesen, C., Erzurum, S. & Kalayci, O. Oxidative stress and antioxidant defense. WAO J. 5, 9–19. https://doi.org/10.1097/WOX.0b013e3182439613 (2012).
Pizzino, G. et al. Oxidative stress: harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 1–13. https://doi.org/10.1155/2017/8416763 (2017).
Pérez-Torres, I. et al. Oxidative stress, plant natural antioxidants, and obesity. Int. J. Mol. Sci. 22, 1786. https://doi.org/10.3390/ijms22041786 (2021).
Dawoud, T. M. et al. Seed priming with pomegranate peel extract improves growth, glucosinolates metabolism and antimicrobial potential of Brassica oleraceae varieties. J. Plant Growth Regulat. 42, 3043–3055. https://doi.org/10.1007/s00344-022-10769-0 (2023).
Limon, R. I., Peñas, E., Martínez-Villaluenga, C. & Frias, J. Role of elicitation on the health-promoting properties of kidney bean sprouts. LWT-Food Sci. Technol. 56, 328–334. https://doi.org/10.1016/j.lwt.2013.12.014 (2014).
Ali, M. et al. Foliar spraying of aqueous garlic bulb extract stimulates growth and antioxidant enzyme activity in eggplant (Solanum melongena L.). J. Integr. Agric. 18, 1001–1013. https://doi.org/10.1016/S2095-3119(18)62129-X (2019).
Chon, S. U., Heo, B. G., Park, Y. S., Kim, D. K. & Gorinstein, S. Total phenolics level, antioxidant activities and cytotoxicity of young sprouts of some traditional Korean salad plants. Plant Foods Hum. Nutr. 64, 25–31. https://doi.org/10.1007/s11130-008-0092-x (2009).
Banerjee, S. et al. Wheatgrass inhibits the lipopolysaccharide-stimulated inflammatory effect in RAW 264.7 macrophages. Curr. Res. Toxicol. 2, 116–127. https://doi.org/10.1016/j.crtox.2021.02.005 (2021).
Hussain, M., Farooq, M. & Lee, D. J. Evaluating the role of seed priming in improving drought tolerance of pigmented and non-pigmented rice. J. Agron. Crop Sci. 203, 269–276. https://doi.org/10.1111/jac.12195 (2017).
Bajwa, A. A., Farooq, M. & Nawaz, A. Seed priming with sorghum extracts and benzyl aminopurine improves the tolerance against salt stress in wheat (Triticum aestivum L.). Physiol. Mol. Biol. Plants. 24, 239–249. https://doi.org/10.1007/s12298-018-0512-9 (2018).
Ghezal, N. et al. Improvement of Pisum sativum salt stress tolerance by bio-priming their seeds using Typha angustifolia leaves aqueous extract. S. Afr. J. Bot. 105, 240–250. https://doi.org/10.1016/j.sajb.2016.04.006 (2016).
Świeca, M., Sęczyk, Ł, Gawlik-Dziki, U. & Dziki, D. Bread enriched with quinoa leaves—The influence of protein–phenolics interactions on the nutritional and antioxidant quality. Food chem. 162, 54–62. https://doi.org/10.1016/j.foodchem.2014.04.044 (2014).
Wakeham, P. The medicinal and pharmacological screening of wheatgrass juice (Triticum aestivum L.): An investigation into chlorophyll content and antimicrobial activity. Univ. Plymouth 6, 20–30 (2013).
Chunthaburee, S., Sanitchon, J., Pattanagul, W. & Theerakulpisut, P. Alleviation of salt stress in seedlings of black glutinous rice by seed priming with spermidine and gibberellic acid. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 42, 405–413. https://doi.org/10.15835/nbha4229688 (2014).
Zimmerli, L. et al. The xenobiotic β-aminobutyric acid enhances Arabidopsis thermotolerance. Plant J. 53, 144–156. https://doi.org/10.1111/j.1365-313X.2007.03343.x (2008).
Zhao, X. et al. Changes in essential oils content, antioxidant capacity and secondary metabolism in different Cinnamomum longepaniculatum varieties. Ind. Crops Prod. 192, 115996. https://doi.org/10.1016/j.indcrop.2022.115996 (2023).
Author information
Authors and Affiliations
Contributions
A.Y.: Experimental, data collection, methodology, writing-original draft. S.S.: supervision, writing-review and editing, funding acquisition. V.Y.: experimental, data collection.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yadav, A., Singh, S. & Yadav, V. Screening herbal extracts as biostimulant to increase germination, plant growth and secondary metabolite production in wheatgrass. Sci Rep 14, 607 (2024). https://doi.org/10.1038/s41598-023-50513-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-50513-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.