Abstract
High seed production makes Sporobolus indicus var. pyramidalis a difficult to control invasive grassland plant. The objective of the present study was to investigate the bioactivity of Cyperus rotundus, Phyllanthus tenellus and Ricinus communis green leaf extracts and of Carica papaya seeds on S. indicus germination without breaking dormancy, simulating the field conditions. The ethanolic extract bioactivity of C. rotundus, P. tenellus, R. communis green leaves and C. papaya seeds, at concentrations of 25, 50 and 75% in S. indicus germination was evaluated. Carotenoids, flavonoids, soluble phenolic compounds and total tannins were quantified in the extracts. The chemical component concentrations varied between alcoholic extracts. The P. tenellus extracts at all dilutions and those of R. communis and C. papaya at 75% completely suppressed S. indicus seed germination at five and ten days which can be attributed to their high tannin concentration, total phenolic compounds and flavonoids.
Similar content being viewed by others
Introduction
The grass Sporobolus indicus var. pyramidalis Beauv1. is an invasive and aggressive nonnative weed, has become a serious threat in many perennial grass pastures distributed in all tropical regions reducing the quality and production of forage crops2.
The S. indicus var. pyramidalis percentage germination is low (6.7–27%)2,3,4 due to the presence of a hard seed coat3. However, the low germination is compensated by high seed production, making it difficult to control. A panicle (30 cm) of S. indicus var. pyramidalis has around one thousand seeds and one plant can produce more than 200 panicles per year5. S. indicus spp. produces more than 1,400 seed per panicle and nearly 45,000 seed per plant3. Smutgrass seed are thought to remain viable for at least 2 years6.
In southern Florida, hexazinone, an expensive herbicide, is the only control option against this plant and it is applied in pastures when infestations of this grass reach 30%5,7,8. In Brazil, S. indicus var. pyramidalis is controlled with glyphosate (360 g L−1) or manually, in small areas when at low densities or in organic and agroecological production systems. In these systems, the plants are ripped, bagged and burned far from the pasture, but its seed reserve in the soil is large. Chemical products to control of S. indicus var. pyramidalis, are expensive or dangerous and its intensive use in integrated systems is a problem. This makes it necessary to develop strategies to manage this plant, including products based on plant extracts, mainly for organic and agroecological production9. In addition, modern agriculture seeks natural organic methods to reduce the extensive and intensive application of chemicals, that impact the environment, public health, and the cost of agricultural production10,11.
Allelochemicals, produced during secondary plant metabolism, may reduce the growth, survival and reproduction of invading species12,13. Phenolic compounds are allelochemicals deriveted the shikimic and acetic acid (polyketide) metabolic pathways in plants14. Cyperus rotundus L. (Cyperaceae), Phyllanthus tenellus Roxb. (Phyllanthaceae), Ricinus communis L. (Euphorbiaceae) and Carica papaya L. (Caricaceae) seeds have toxicological properties. Gallic acid, chlorogenic acid, 3,4-dihydroxybenzaldehyde, p-hydroxybenzoic acid, catechol, tannic acid, ricinine are some of the allelochemical phenolic compounds found in these species15. But the allelopathic potential of these plants on seeds weeds needs to be better studied10. Aqueous extracts have been studied16,17,18, but many non-polar bioactive substances cannot be dissolved by water at room temperature, unlike organic solvents13. Polar solvents such as methanol, ethanol, acetone, or acetonitrile give much high extraction efficiencies14.
Phenolic compounds, originated to protect plants from oxidative damage, are also involved in plant allelopathy inducing changes in membrane permeability, inhibition of nutrient uptake, cell division, stretching and submicroscopic structure, altering enzyme activity, respiration, and synthesis of hormones and proteins14. Studies on the performance of phenolic compounds such as allelopaths can provide data to development sustainable methods of agriculture, forestry, natural resources and conservation of the environment.
The objective of the present study was to evaluate the bioactivity of alcoholic extracts of C. rotundus, P. tenellus, R. communis green leaves and C. papaya seeds with on the S. indicus var. pyramidalis germination without breaking dormancy, simulating the field conditions.
Results
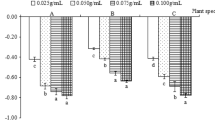
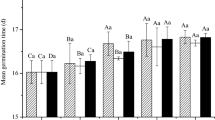
The concentration of the chemical components varied between and among the alcohol extracts of C. rotundus, P. tenellus and R. communis green leaves and that of C. papaya seeds. Total flavonoids ranged from 0.32 (P. tenellus to 25%) to 10.00 mg 100 g−1 (C. rotundus to 75%). Total tannins from 0.24 (C. papaya 25%) to 16.32 mg catechin g−1 (P. tenellus 75%). Soluble phenolic compounds from 0.89 (25% P. tenellus) to 754.23 mg kg−1 (C. papaya 75%). Total carotenoids from 0.09 (R. communis at 25%) to 7.00 mg 100 g−1 (C. papaya at 75%) (Table 1). P. tenellus extracts at all dilutions and those of R. communis at 75% and C. papaya extracts completely suppressed S. indicus seed germination up to 30 days after aplication (Table 2).
Discussion
The variation in the concentration of chemical components between the alcoholic extracts confirms their wide occurrence and diversity in plants19,20 as reported for Artemisia campestres L. (Asteraceae), A. Herba halba L. (Asteraceae), A. arboresens L. (Asteraceae), A. arvensis L. (Asteraceae), Juniperus oxycedrus L. (Cupressaceae), Globularia alypum L. (Globulariaceae), Oudneya africana R. Br. (Brassicaceae), Monuta Route L. (Rutaceae), Thapsia garganica L. (Apiaceae), Thymelaea hirsuta L. (Thymelaeaceae) and Teucrium polium L. (Lamiaceae)21 and thirty-two other herbs22. This makes plants from different habitats in Sardinia, Italy23 of the same species growing in different conditions24 have chemical composition variation as reported for Myrtus communis L. (Myrtaceae). The variation in the concentration of the chemical components between the alcoholic extracts is due to their proportions in the solvent/solute (dilution) which determines the effectiveness of the plant extracts and the isolated compounds25. In addition to dilution, the solvent may also alter the chemical composition of the extracts, as reported for Origanum vulgare L. (Lamiaceae)26, Anthocleista grandiflora Gilg. (Gentianaceae) and Combretum erythrophyllum Burch. (Combretaceae) in wich compound quantity and diversity varied according to the extractors and their concentration25.
Flavonoids, a group of phenolic compounds resulting from secondary metabolism, are widely found in plants27 and their higher amount in the 75% C. rotundus extract agrees with that reported for the rhizome extract of this plant28,29,30,31. However, abiotic and biotic stress26 and changes in seasonal dynamics can affect compound content and when it is higher in the plant it will also be in the extract, as reported for Dryopteris erythrosora (DC Eaton) Kuntze (Dryopteridaceae) with the transport of flavonoids from the leaves to the stem in the growing season, comprising summer (26.9 °C) and early autumn (16.9 °C) in Shanghai, China32. The highest total tannin levels (another phenolic compound group) from the P. tenellus extract could be a response to the stressful environment in which this invasive species was collected33, area with stones, few soil and water deficit, in the microregion of Campina Grande, Paraíba, Brazil, with few soil and water deficits. The tannin accumulation, in this case, has an antioxidative function34 and agrees with the phytochemical profile of the methanolic solution (80%) of the whole P. tenellus plant35. The highest phenolic compound content and total carotenoids in the C. papaya L. seed extract is due to its function in sanity and resistance to pests and diseases, as a strategy for seed survival36, mainly against oxidative stress37. These compunds act in response to environmental stress conditions protecting against injuries, as reported in the identification of the phenolic profile of papaya fruits36,38. Secondary products of metabolism such as flavonoids, tannins, phenolic compounds and carotenoids39, may act in inhibiting germination40 reducing tissue growth or causing death by increasing cell membrane permeability, as reported for Cucumis sativus L. (Cucurbitaceae)41, Lactuca sativa L. (Asteraceae)14, Phaseolus vulgaris L. (Fabaceae)42 resulting in the inhibition of radicular elongation and ultra structural changes and cell division.
The suppression of S. indicus germination by C. papaya, P. tenellus and R. communis extracts may be due to their high tannin concentration and total phenolic compounds (derived from the acetate and shikimic acid route or their combination)39. These compounds bind strongly to proteins by hydrogen bonds and hydrophobic interaction, deactivating them and blocking germination metabolism43,44 or preventing the access of free oxygen to the embryo and the release of carbon dioxide45. This was reported for Sorghum bicolor L. Moench. (Poaceae) which tannin content was correlated with its germination. The highest flavonoid concentration in C. rotundus, R. communis and C. papaya extracts at 75%, also explains the allelopathic effect on S. indicus germination. In addition, the flavonoids are compounds with high antioxidant power46 suppressing germination by inhibiting the indole-acidase oxidase (IAA oxidase), gibberellic acid (GA3) and indo-3-acetic acid (IAA)47. The allelopathic effect of Dittrichia viscosa (L.) W. Greuter extracts was attributed to flavonoids48 and, even at low concentrations (0.1–1.0%), those of Ocimum gratissimum L. (Lamiaceae) inhibited the germination and growth of corn and beans49. The suppressive germination effect by the C. papaya extract at 75% may also be due to caricacin50, which suppresses cell division and phytohormone production and increases the permeability of membranes, inhibiting germination43,51. The absence of toxicity of the R. communis extract at 25 and 50% can be explained by their adsorption by allelopathic active compounds such as sugars and other S. indicus seed carbohydrates, whereas this was not sufficient at 75% concentration due to the high concentration. High sugar concentrations as well as of other carbohydrates, such as glucose and fructose, maltose, sucrose, raffinose, myo-inositol and galactinol have been reported for Poa annua L.52, Melinis minutiflora P. Beauv. (Poaceae), Echinolaena inflexa Poir. (Poaceae), and Lolium multiflorum L. (Poaceae)53 from the same S. indicus family. Ricin, a highly toxic R. communis heterodimeric protein is composed of polypeptide chains with an affinity for cell surface carbohydrates54,55,56,57 becomes inert when adsorbed by them thereby not influencing germination18,58,59. Lectin, a N-acetylgalactosamine present in seeds, including those of the Poaceae family60 is another protein class with reversible carbohydrate binding capacity that can adsorb ricin and other allelopathic compounds, deactivating the R. communis extract atby inhibiting the germination of the invasive plant S. indicus var. pyramidalis.
Conclusion
Phyllanthus tenellus alcohol extracts at all R. communis concentrations and C. papaya, at 75%, suppressed the germination of S. indicus var. pyramidalis. These extracts have the potential to manage this plant in organic and agroecological production systems.
Material and Methods
Raw material, preparation and characterization of extracts
Extracts were obtained from C. rotundus, P. tenellus and R. communis green leaves and C. papaya seeds by immersion in 70% ethyl alcohol for seven days14. The alcohol was extracted at 250 °C and the extract filtered and diluted in distilled water to obtain the concentrations of 25, 50 and 75% and their effects were compared with distilled water (control). The chemical composition of extracts at all concentrations was characterized.
Soluble phenolic compounds
The extracts were prepared by adding 10 mL of methanol: acetic: water solution (50:3.7:46.3) to 10 mg of extract, sonicated for 15 min and centrifuged (NT810 model, Nova Técnica Ind. Com. Equipamentos para Laboratório LTDA, Brazil) at 16,000 rpm min−1 for 15 min. An aliquot of the extract (0.2 mL) was withdrawn and 1:10 (v/v) Folin-Ciocalte: water solution added. The final solution was incubated for 10 min at room temperature14. A total of 0.8 ml of sodium carbonate (7.5%) was added to the resulting solution, which was mixed and incubated for 30 min at room temperature. Soluble phenolic compound concentrations were determined using UV-Vis spectrophotometer (4001/4 model, Spectronic® 20 GenesysTM, USA) at 473 nm with gallic acid as standard.
Total flavonoids
Flavonoids were extracted with ethyl alcohol solution (95%) - HCl (1.5 N) at the ratio 85:1515. An aliquot of 10 mL of the extract solution was added to 1.0 g of the alcoholic extract. The samples were vortexed for 2 min and the contents packed in amber flasks for 24 h at 4 °C. After 24 h, the material was centrifuged at 3,500 rpm (2,380 × g) for 10 min and the supernatant removed. The volume was completed to 10 ml and readings were performed using a UV-Vis spectrophotometer (4001/4 model, Spectronic® 20 GenesysTM, USA) at 374 nm with the results expressed in mg 100 g−1.
Total carotenoids
Total carotenoids were extracted in a steel vessel with an aliquot of 2.0 g of alcoholic extract, 6.0 mL of isopropyl alcohol and 2.0 mL of hexane stirred for 2 min61. The contents were transferred to an amber 125 mL separatory funnel, making up the volume with water. After 30 min resting, the material was washed, repeating the operation three times. The contents were filtered with powdered cotton wool with anhydrous sodium sulfate into a 10 mL volumetric flask wrapped with aluminum with 2.0 mL of acetone and the volume made up with hexane. The readings were performed in a UV-Vis spectrophotometer at 450 nm and the results expressed in mg 100 g−1.
Total tannins
The samples of alcoholic extracts were allowed to stand for 1 h in 40 mL of 50% methyl alcohol, centrifuged at 15,000 rpm for 15 min and the supernatant transferred to a 100 mL volumetric flask. A 70% acetone solution was added to the precipitate, which was kept standing for a further 1 h. The mixture was again centrifuged at 15,000 rpm for 15 min and the supernatant discarded. The precipitate was placed in a thermostatic bath at 100 °C for 3 h, cooled in an ice bath, filtered into a 50 ml volumetric flask and the volume filled with the extractive solution. The readings were made in 6 mL aliquots of butanol: HCl and 0.2 mL of 2 N:FeNH4(SO4).12H2O per test tube. After stirring, these tubes were placed in a thermostatic bath at 100 °C for 50 min and cooled in an ice bath. The reading was performed in a UV-Vis spectrophotometer at 550 nm and the results expressed in mg of catechin g−1.
Panicles collection
Panicles without evidence of herbivory and fungi and with mature seeds were collected from plants distributed in ten (10) farms with pastures infested by S. indicus var. pyramidalis in the state of Paraíba, northeastern Brazil. Mature seeds were randomly selected and naturally dried. The viability test was performed in duplicate in batches of 100 seeds of each property. The viability test was performed for 30 days62. However, seeds that did not germinate within 10 days were rotting4.
Bioassay
S. indicus germination was evaluated in triplicate with 100 seeds every 10 days in a germination box (Gerbox®) (11 × 11 × 3.5 cm) with two germination paper (Germitest®) moistened with 18 ml of the different extracts and distilled water in the control. Seed dormancy were not broken to simulate field conditions. The germination assays were done in a germination chamber at 20 °C with 14 h light per daily. Germination was evaluated daily by 10 days4. Seeds with radicle protrusion were considered germinated. The percentage of germination was obtained with the formula: % G = (N/A)*100, where: N = total number of seeds germinated; A = total number of seeds placed to germinate63,64,65,66,67.
Experimental design and statistical analysis
The experimental design was completely randomized with three replicates of 100 seeds. The germination rates was compared across independent samples by using non-parametric Kruskal Wallis H test. Further, Mann Whitney U test was used to compare the two germination rates.
References
Powell, G., Tosh, C. R. & Hardie, J. Host plant selection by aphids: behavioral, evolutionary, and applied perspectives. Annu. Rev. Entomol. 51, 309–330, https://doi.org/10.1146/annurev.ento.51.110104.151107 (2006).
Rana, N., Wilder, B., Sellers, B., Ferrell, J. & MacDonald, G. Effects of environmental factors on seed germination and emergence of smutgrass (Sporobolus indicus) varieties. Weed Sci. 60, 558–563, https://doi.org/10.1614/WS-D-11-00208.1 (2012).
Currey, W.L.R., Parradoand, D. W J. Seed characteristics of smutgrass. Pages 53–54 in Proceedings of the 32nd Soil Crop Science Society of Florida. Gainesville, FL: Soil and Crop Science Society of Florida, (1973).
Guido, A. C. & Pillar, D. H. V. D. Exploring seed to seed effects for understanding invasive species success. Perspect. Ecol. Conser. 15, 234–238, https://doi.org/10.1016/j.pecon.2017.07.006 (2017).
Ferrell, J. A., Mullahey, J. J., Dusky, J. A. & Roka, F. M. Competition of giant smutgrass (Sporobolus indicus) in a bahiagrass pasture. WSSA. 54, 100–105, https://doi.org/10.1614/WS-05-061R1.1 (2006).
McCaleband, J. E. E. & Hodges, M. Smutgrass control at Range Cattle Station, Ona, Florida. Pages 182–186 in Proceedings of the 24th Southern Weed Science Society. Southern Weed Science Society (1971).
Quattrocchi, U. CRC world dictionary of grasses: common names, scientific names, eponyms, synonyms, and etymology. 2383p (Boca Raton: CRC Press, 2006)..
Rana, N. et al. Impact of soil pH on bahiagrass competition with giant smutgrass (Sporobolus indicus var. pyramidalis) and small smutgrass (Sporobolus indicus var. indicus). WSSA. 61, 109–116, https://doi.org/10.1614/WS-D-12-00070.1 (2013).
Jabran, K., Mahajan, G., Sardana, V. & Chauhan, B. S. Allelopathy for weed control in agricultural systems. J. Crop. Prot. 72, 56–75, https://doi.org/10.1016/j.cropro.2015.03.004 (2015).
Musa, D. D., Esson, A. E., Shuaibu, B. U. & Adebola, M. I. Allelopathic effect of Senna obtusifolia on the germination and growth of cowpea and maize. Afr. J. Plant. Soil. Res. 5, 71–74 (2016).
Hidangmayum, A. & Sharma, R. Effect of different concentration of commercial seaweed liquid extract of Ascophylum nodosum on germination of onion (Allium cepa L.). J. Pharmacogn. Phytochem. 6, 1488–1481 (2017).
Zheng, Y. L. et al. Integrating novel chemical weapons and evolutionarily increased competitive ability in success of a tropical invader. N. Phytol. 205, 1350–1359, https://doi.org/10.1111/nph.13135 (2015).
Cheng, F. & Cheng, Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant. Sci. 6, e01020, https://doi.org/10.3389/fpls.2015.01020 (2015).
Li, Z. H., Wang, Q., Ruan, X., Pan, C. D. & Jiang, D. A. Phenolics and plant allelopathy. Molecules 15, 8933–8952, https://doi.org/10.3390/molecules15128933 (2010).
Singh, R. K. & Geetanjali, G. Phytochemical and pharmacological investigations of Ricinus communis Linn. Alg. J. Nat. Prod. 3, 120–129 (2015).
Li, Y. C., Guo, Q. S., Shen, H. J., Fang, H. L. & Redai, Y. Z. X. Preliminary isolation and identification of allelopathic compounds from Jatropha curcas L. fruit shells. J. Trop. Subtrop. Bot. 21, 73–77 (2013).
Singh, R. Geetanjali. Phytochemical and Pharmacological Investigations of Ricinus communis Linn. Alg. J. Nat. Prod. 3, 120–129 (2015).
Marwat, S. K. et al. Ricinus communis: Ethnomedicinal uses and pharmacological activities. Pak. J. Pharm. Sci. 30, 1815–1827 (2017).
Hrazdina, G., Borzel, A. J. & Robinson, W. B. Studies on the stability of the anthocyanidin-3,5- diglucosides. Am. J. Enol. Viticult. 21, 201–204 (1970).
Figueiredo, A. C., Barroso, J. G., Pedro, L. G. & Scheffer, J. J. C. Factors affecting secondary metabolite production in plants, volatile components and essential oils. Flavour. Frag. J. 23, 213–226, https://doi.org/10.1002/ffj.1875 (2008).
Djeridane, A. et al. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 97, 654–660, https://doi.org/10.1016/j.foodchem.2005.04.028 (2006).
Wojdyło, A., Oszmiański, J. & Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 105, 940–949, https://doi.org/10.1016/j.foodchem.2007.04.038 (2007).
Petretto, G. L. et al. Variability of chemical composition and antioxidant activity of essential oils between Myrtus communis var. Leucocarpa DC and var. Melanocarpa DC. Food Chem. istry 197, 124–131, https://doi.org/10.1016/j.foodchem.2015.10.056 (2016).
Sulimaa, P., Krauze-Baranowska, M. & Przyborowskia, J. A. Variations in the chemical composition and content of salicylic glycosides in the bark of Salix purpurea from natural locations and their significance for breeding. Fitoterapia 118, 118–125, https://doi.org/10.1016/j.fitote.2017.03.005 (2017).
Eloff, J. N. Which extractant should be used for the screening and isolation of antimicrobial components from plants? J. Ethnopharmacol. 60, 1–8, https://doi.org/10.1016/S0378-8741(97)00123-2 (1998).
Teixeira, B. et al. Chemical composition and bioactivity of different oregano (Origanum vulgare) extracts and essential oil. J. Sci. Food Agric. 30, 2707–2714, https://doi.org/10.1002/jsfa.6089 (2013).
Manoj, G. S. & Murugan, K. Phenolic profiles, antimicrobial and antioxidant potentiality of methanolic extract of a liverwort, Plagiochila beddomei Steph. Indian. J. Nat. Prod. Resour. 3, 173–183 (2012).
Sunil, A. G. et al. Total oligomeric flavonoids of Cyperus rotundus ameliorates neurological deficits, excitotoxicity and behavioral alterations induced by cerebral ischemic-reperfusion injury in rats. Brain Res. Bull. 84, 394–405 (2014).
Kumar, K. H., Razack, S., Ilaiyaraja, N. & Khanum, F. Phytochemical analysis and biological properties of Cyperus rotundus L. Ind. Crop. Prod. 52, 815–826, https://doi.org/10.1016/j.indcrop.2013.11.040 (2014).
Kumar, K. H., Tamatam, A., Pal, A. & Khanum, F. Neuroprotective effects of Cyperus rotundus on SIN-1 induced nitric oxide generation and protein nitration: ameliorative effect against apoptosis mediated neuronal cell damage. Neurotoxicology 34, 150–159, https://doi.org/10.1016/j.neuro.2012.11.002 (2013).
Kandikattua, H. K. et al. LC–ESI-MS/MS analysis of total oligomeric flavonoid fraction of Cyperus rotundus and its antioxidant, macromolecule damage protective and antihemolytic effects. Pathophysiology 22, 165–173, https://doi.org/10.1016/j.pathophys.2015.07.001 (2015).
Xie, Y. et al. Seasonal dynamics of total flavonoid contents and antioxidant activity of Dryopteris erythrosora. Food Chem. 186, 113–118, https://doi.org/10.1016/j.foodchem.2014.05.024 (2015).
Silva, T. C. L. et al. Estudo da toxicidade subcrônica de Phyllanthus tenellus Roxb: avaliação comportamental. Rev. Enferm. 1, 17–22, https://doi.org/10.5205/0201200803 (2008).
Williams, R. J., Spencer, J. P. E. & Rice, C. E. Flavonoids: Antioxidants or signaling molecules? Free. Radic. Biol. Med. 36, 838–849, https://doi.org/10.1016/j.freeradbiomed.2004.01.001 (2004).
Komuraiah, A. et al. Antibacterial studies and phytochemical constituents of South Indian Phyllanthus species. Afr. J. Biotechnol. 8, 4991–4995 (2009).
Imani, A., et al. Seed Sci Technol. 39, 204-207 (2011).
Tommasi, F., Paciolla, C., Pinto, M. C. & Gara, L. A. Comparative study of glutathione and ascorbate metabolism during germination of Pinus pinea L. seeds. J. Exp. Bot. 52, 1647–1654 (2001).
Rivera-Pastrana, D. M., Yahia, E. M. & González-Aguilar, G. A. Phenolic and carotenoid profiles of papaya fruit (Carica papaya L.) and their contents under low temperature storage. J. Sci. Food Agric. 90, 2358–2365, https://doi.org/10.1002/jsfa.4092 (2010).
Yang, S. et al. Chemical constituents of Cinnamomum septentrionale leaf litter and its allelopathic activity on the growth of maize (Zea mays). Nat. Prod. Res. 31, 1314–1317, https://doi.org/10.1080/14786419.2016.1236102 (2017).
Šežiene, V., Baležentienė, L. & Maruška, A. Identification and allelochemical activity of phenolic compounds in extracts from the dominant plant species established in clear-cuts of Scots pine stands. iForest. 10, e1–e6, https://doi.org/10.3832/ifor1791-009 (2017).
Li, H. H., Inoue, M., Nishimura, H., Mizutani, J. & Tsuzuki, E. Interaction of trans-cinnamic acid, its related phenolic allelochemicals, and abscisic-acid in seedling growth and seed-germination of lettuce. J. Chem. Ecol. 19, 1775–1787, https://doi.org/10.1007/BF00982307 (1993).
Cruz, O. R., Anaya, A. L. & Hernandez-Bautista, B. E. Effects of allelochemical stress produced by sicyosdeppei on seedling root ultrastructure of Phaseolous valgaris and Cucubita ficifolia. J. Chem. Ecol. 24, 2039–2057 (1998).
Sartor, L. R., Chini, P. F. A. N., Martin, T. N., Marchese, J. A. & Soares, A. B. Alelopatia de acículas de Pinus taeda na germinação e no desenvolvimento de plântulas de Avena strigosa. Ciênc. Rural. 39, 1653–1659, https://doi.org/10.1590/S0103-84782009000600004 (2009).
Parr, A.J. & Bolwell, G.P. Phenols in the plant and in man. The potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. J. Agric Food Chem. 80, 985–1012. 10.1002/(SICI)1097-0010(20000515)80:7<985::AID-JSFA572>3.0.CO;2-7 (2000).
Weidner, S. et al. Analysis of phenolic compounds and antioxidant abilities of extracts from germinating Vitis californica seeds submitted to cold stress conditions and recovery after the stress. Int. J. Mol. Sci. 15, 16211–16225, https://doi.org/10.3390/ijms150916211 (2014).
Dridi, A., Hadef, Y. & Bouloudani, L. Determination of total phenol, flavonoid, antioxidant and antimicrobial activity of methanolic extract of Teucrium polium L. in Algerian East. J. Pharmacogn. Phytochem. Res. 8, 1566–1570 (2016).
Hinderer, W., Petersen, M. & Seitz, H. U. Inhibition of flavonoid biosynthesis by gibberellic acid in cell suspension cultures of Daucus carota L. Planta 160, 544–549, https://doi.org/10.1007/BF00411143 (1984).
Grande, M., Piera, F., Cuenca, A., Torres, P. & Bellido, I. Flavonoids from Inula viscosa. Planta Medica 51, 414–419, https://doi.org/10.1055/s-2007-969536 (1985).
Macdonald, I. O., Oludare, A. S. & Olabiyi, A. Phytotoxic and anti-microbial activities of flavonoids in Ocimum gratissimum. Life Sci. 7, 45–48 (2010).
Reyes, M. N., Perez, A. & Cuevas, J. Detecting endogenous growth regulators on the sarcotesta, sclerotesta, endosperm and embryo by paper chromatography on fresh and old seeds of two papaya varieties. J. Agr. U Puerto Rico 64, 164–172 (1980).
Karakaya, S. Bioavailability of phenolic compounds. Crit. Rev. Food Sci. Nutr. 44, 453–464 (2004).
Kellmann-Sopyła, W., Lahuta, L. B., Giełwanowska, I. & Górecki, R. J. Soluble carbohydrates in developing and mature diaspores of polar Caryophyllaceae and Poaceae. Acta Physiol. Plant. 37, e118, https://doi.org/10.1007/s11738-015-1866-z (2015).
Souza, A., Sandrin, C. Z., Moraes, M. G. & Ribeiro, R. C. L. F. Diurnal variations of non-structural carbohydrates in vegetative tissues of Melinis minutiflora, Echinolaena inflexa and Lolium multiflorum (Poaceae). Braz. J. Bot. 28, 755–763, https://doi.org/10.1590/S0100-84042005000400010 (2005).
Deeks, E. D. et al. The low lysine content of ricin A chain reduces the risk of proteolytic degradation after translocation from the endoplasmic reticulum to the cytosol. Biochem. 41, 3405–3413 (2001).
Lord, M. J. et al. Ricin. Mechanism of cytotoxicity. Toxicol. Rev. 22, 53–64 (2003).
Barbieri, L. et al. Enzymatic activity of toxic and non-toxic type 2 ribosome-inactivating proteins. FEBS Lett. 563, e00286–8, https://doi.org/10.1016/S0014-5793(04)00286-8 (2004).
Jasheway, K., Pruet, J., Anslyn, E. V. & Robertus, J. D. Structure-based design of ricin inhibitors. Toxins 3, 1233–1248, https://doi.org/10.3390/toxins3101233 (2011).
Etzler, M. E. et al. A Nod factor binding lectin with apyrase activity from legume roots. Proc. Natl. Acad. Sci. USA 96, 5856–5861 (1999).
Kalsi, G. & Etzler, M. E. Localization of a Nod factor-binding protein in legume roots and factors influencing its distribution and expression. Plant. Physiol. 124, 1039–1048, https://doi.org/10.1104/pp.124.3.1039 (2000).
Rüdiger, H. & Gabius, H. J. Plant lectins: occurrence, biochemistry, functions and applications. Glycoconj. J. 18, 589–613 (2001).
Bhadoria, P. B. S. Allelopathy: a natural way towards weed management. Am. J. Agric. Exp. 1, 7–20 (2011).
Brasil. Ministério da Agricultura, Pecuária e Abastecimento. Regras para análise de sementes. 399p (Brasília: Mapa/ACS, 2009).
Betts. J. & Officer, D. Control of giant parramatta grass: Agnote DPI/354, Orange: NSW Agriculture, 11 p. (2001).
Fu, M., Feng, H. J., Chen, Y., Wang, D. B. & Yang., G. Z. Antioxidant activity of Garcinia xanthochymus leaf, root and fruit extracts in vitro. Chin. J. Nat. Medicines. 10, 129–134 (2012).
Francis, F. J. Analysis of anthocyanins. In: Markakis, P. Anthocyanins as food colors. (p.181–206). London: Academic Press (1982).
Higby, W. K. A simplified method for determination of some the carotenoid distribution in natural and carotene fortified orange juice. J. Food Sci. 27, 42–49, https://doi.org/10.1111/j.1365-2621.1962.tb00055.x (1962).
Dixon, R. A. & Paiva, N. L. Stress-induced phenylpropanoid metabolism. Plant. Cell 7, 1085–1097, https://doi.org/10.1105/tpc.7.7.1085 (1995).
Acknowledgements
To “Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) for financial support.
Author information
Authors and Affiliations
Contributions
W.S.R., R.L.A.B., E.U.A. and J.F.S.M. designed the research; J.F.S.M., L.S.R. and W.S.R. performed the experiments; W.S.R., J.C.Z., R.L.A.B., K.P.L., A.P.A., F.B.C. and J.F.S.M. wrote the manuscript. All authors approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Macêdo, J.F.d.S., Ribeiro, L.S., Bruno, R.d.L.A. et al. Green leaves and seeds alcoholic extract controls Sporobulus indicus germination in laboratory conditions. Sci Rep 10, 1599 (2020). https://doi.org/10.1038/s41598-020-58321-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-58321-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.