Abstract
Solute carrier transporters are integral membrane proteins, and are important for diverse cellular nutrient transports, metabolism, energy demand, and other vital biological activities. They have recently been implicated in pancreatic cancer and other cancer metastasis, angiogenesis, programmed cell death and proliferation, cell metabolism and chemo-sensitivity. Here we report the study of 13 human solute carrier membrane transporters using the highly accurate AlphaFold2 predictions of 3D protein structures. In the native structures, there are hydrophobic amino acids leucine (L), isoleucine (I), valine (V) and phenylalanine (F) in the transmembrane alpha-helices. These hydrophobic amino acids L, I, V, F are systematically replaced by hydrophilic amino acids glutamine (Q), threonine (T) and tyrosine (Y), thus the QTY code. Therefore, these QTY variant transporters become water-soluble without requiring detergents. We present the superposed structures of these native solute carrier transporters and their water-soluble QTY variants. The superposed structures show remarkable similarity with RMSD ~ 1 Å–< 3 Å despite > 46% protein sequence substitutions in transmembrane alpha-helices. We also show the differences of surface hydrophobicity between the native solute carrier transporters and their QTY variants. Our study may further stimulate designs of water-soluble transmembrane proteins and other aggregated proteins for drug discovery and biotechnological applications.
Similar content being viewed by others
Introduction
It is well-known that pancreatic cancer is a highly fatal disease1,2,3. Many active basic and clinical research efforts have been made, but no effective treatment is yet in sight. Recently, several solute carrier (SLCs) transporters have come to light that are involved in six crucial routes of pancreatic cancer resulting in very poor prognoses including: (i) cancer cell proliferation, (ii) programmed cell death, (iii) invasion and metastasis, (iv) angiogenesis, (v) cellular metabolism and (vi) chemo-sensitivity. These SLC transporters move a broad range of key substrates including (a) amino acids, (b) nucleotides, (c) essential metal ions including zinc, magnesium, sodium, lithium, copper, other organic ions, NH4+, HCO3−, (d) short chain fatty acids, (e) co-factors, vitamins, and (f) other organic ions and compounds into cells. Some of these SLCs are involved in pancreatic cancer metastasis and angiogenesis, others are involved in proliferation and programmed cell death, as well as cellular metabolism and chemo-sensitivity (Table 1)1,2,3. With these new insights, these SLCs not only should be the focus of intense research, but should also be the targets for new therapeutic strategies to treat pancreatic cancer and perhaps other cancer metastasis1,2,3,4.
There are 46 distinct gene families of SLC transporters comprising 384 genes in the human genome2,5. Some members of these SLC transporters have recently been found to be involved in several key aspects of cancer including fatal pancreatic cancer (Table 1). One of the key characteristics of cancer is deregulation of cellular energetics, namely the insatiable energy demand including sugars and nutrients through upregulating the transporters1,3,6. By effectively blocking the key logistics of transport systems to the cancer cells, we may be able to add another tool to more effectively treat caners.
SLC transporters are involved in multiple cellular pathways for pancreatic cancer and others in a single pathway. For example, several are involved in metastasis, programmed cell death, proliferation, chemo-sensitivity (Table 1) including SLC2A1 (also called GLUT1)7,8,9, SLC7A1110, SLC4A411, SLC1A512 and SLC7A513,14, SLC39A615,16. On the other hand, others are only involved a single pathway (Table 1) including SLC39A317,18,19, SLC9A120,21,22, SLC6A1423, SLC4A724, SLC5A825,26 and SLC41A127. The motivation of this study is to endeavor to discover new drugs and to generate therapeutic monoclonal antibodies, by targeting those that are involved in multiple pathways.
SLC transporters have multiple transmembrane (TM) helices. Most have 10TM-12TM helices depending on their transporting substrates with the longest having 13TM (SLC5A8 for short chain fatty acids). Several of these SLC transporters have large N-termini and extracellular loops (Figs. 1, 2) of ~ 30–60 amino acids. Those with large extracellular loops are more likely to be better targets for generating therapeutic monoclonal antibodies since these extracellular loops may stimulate robust immune responses during immunizations.
Protein alignments of six solute carrier transporters with their QTY variants. The symbols | and * indicate the identical and different amino acids, respectively. Please note the Q, T and Y amino acid replacement (red). The alpha-helices (blue) are shown above the protein sequences. The loop color codes are: internal (yellow) and external (red). Features of natural and QTY variants are: molecular weight, pI, total variation % and transmembrane variation %. The alignments are: (a) SLC2A1 versus SLC2A1QTY, (b) SLC7A11 versus SLC7A11QTY, (c) SLC4A4 versus SLC4A4QTY, (d) SLC1A5 versus SLC1A5QTY, (e) SLC7A5 versus SLC7A5QTY, (f) SLC29A1 versus SLC29A1QTY. Although there are significant QTY changes for the TM alpha-helix changes, > 45% for SLC29A1, > 50% for SLC7A11, their changes of molecular weight and pI are insignificant (Table 2).
Protein alignment of eight native solute carrier transporters with their water-soluble QTY variants. All characteristics and color codes are referred in Fig. 1. The alignments are: (a) SLC39A6 versus SLC39A6QTY, (b) SLC39A3 versus SLC39A3QTY, (c) SLC9A1 versus SLC9A1QTY, (d) SLC6A14 versus SLC6A14QTY, (e) SLC4A7 versus SLC4A7QTY, (f) SLC5A8 versus SLC5A8QTY, (g) SLC41A1 versus SLC41A1QTY (see Tables 2, 3).
On 15 July 2021, Google DeepMind announced the AlphaFold2, and at the same time David Baker’s lab introduced RoseTTAFold as machine learning revolutionary tool for the very accurate prediction of protein structures28,29,30. AlphaFold2 and, to a lesser extent, RoseTTAFold, have already made an enormous impact on our understanding of 350,000 protein structures. On July 28, 2022, DeepMind released 214 million protein structures, nearly all known protein structures. AlphaFold2 predicts the structures with very high accuracy for 35% of all protein structures (~ 75 million), and with high confidence for another 45% (~ 96.3 million). AlphaFold2 has truly started a new era of digital biology. Nevertheless, academic investigators, the pharmaceutical and biotech companies must still ultimately study the physical structures of proteins, including SLC membrane transporters since the structures are vital to understanding how substrates including sugars, amino acids, essential ions, organic molecules, drugs, or other essential nutrients are transported across highly regulated and controlled cell membranes.
We previously applied the QTY (Glutamine, Threonine, Tyrosine) code to design several detergent-free transmembrane (TM) protein chemokine receptors and cytokine receptors for various uses using conventional computing programs to simulate several G protein-coupled receptors. Each took ~ 5 weeks to complete the simulation33,34,35. The expressed and purified water-soluble proteins exhibited predicted characteristics and retained ligand-binding activity31,32,33,34,35. In July 2021, we prepared QTY variant protein structure predictions using AlphaFold2, achieving better results in 1–2 hours36,37, rather than ~ 5 weeks for each molecular simulation using GOMoDo, AMBER and YASARA programs31,32,33. We also produced a program and website for generating the membrane protein water-soluble QTY variants38.
Here, we report using AlphaFold2 to design water-soluble QTY variants of the 13 solute carrier transporters, and to directly compare with their counterpart native structures. In addition to targeting the key nutrients and ion uptake activity of cancer cells, these QTY variant water-soluble SLC transporters can prospectively find many additional applications. Working with water-soluble QTY variants may substantially accelerate the discovery and development of therapeutic and diagnostic biologicals.
Results and discussions
Protein sequence alignments and other characteristics
We aligned the native SLC transporters with their QTY variants. Despite significant QTY replacement of hydrophobic residues in the transmembrane domains (~ 44–55%) in the SLC transporters, the isoelectric focusing point pI and molecular weight remain rather similar (Figs. 1 and 2, Table 2). This is because Q, T, Y amino acids do not introduce any charges, they only introduce water-soluble side chains. Q (glutamine) side chains form 4 water hydrogen bonds, 2 donors through –NH2, and 2 acceptors through oxygen on –C=O; the sidechains –OH of T (threonine) and Y (tyrosine) form 3 water hydrogen bonds, 1 donor from H (hydrogen) and 2 acceptors from O (oxygen).
Since the electron density maps share remarkable structure similarities between leucine (L) versus glutamine (Q); isoleucine (I), valine (V) versus threonine (T); and phenylalanine (F) versus tyrosine (Y), the QTY code selects 3 neutrally polar amino acids: glutamine, threonine and tyrosine to replace 4 hydrophobic amino acids leucine, isoleucine, valine and phenylalanine. After applying the QTY code, the hydrophobic amino acids in the transmembrane segments are replaced by Q, T, and Y, therefore the transmembrane segments have significantly reduced hydrophobicity. For example, SLC39A3 and SLC29A1 differ > 54 and > 45% from their water-soluble QTY variants, respectively, in their transmembrane alpha-helical segments. (Figs. 1, 2, Table 2).
Other characteristics are also notable. The pIs (isoelectric-focusing points) vary, some in the acidic and some in the basic range. For example, native SLC7A11 has a basic pI of 9.29. On the other hand, SLC1A5 has an acidic pI of 5.34. Others including SLC9A1 have a near neutral pI of 6.74 (Table 2). It is noted that the pIs are identical for the native and QTY variants for SLC4A4 (pI 6.35), SLC39A3 (pI 6.39), SLC4A7 (pI 6.26), and SLC41A1 (pI 5.11) despite the large number of QTY substitutions. The reason is that Glutamine, Threonine, Tyrosine (Q, T, Y) have neither positive nor negative charges at neutral pH. Therefore, substitutions of Q, T, Y do not change the pIs. This is significant because altered pIs could cause non-specific interactions.
Moreover, while there are between > 45– > 54% QTY substitutions in the transmembrane helices, the molecular weights of the native and QTY variants differ by only a few hundreds of Daltons. This is due to a) the substitutions of CH3- on Lue and Val, by -OH groups to Gln (Q) and Thr (T), and b) the addition of OH- on Tyr (Y). These increase the protein molecular weights (Figs. 1, 2, Table 2).
Superposition of native transporters and their water-soluble QTY variants
In our current study, the native SLC structures determined by X-ray crystal or CryoEM were superimposed and compared to their QTY variants. The molecular structures of native SLC transporters are already available for SLC2A1 (PDB: 6THA)39, SLC7A11 (PDB: 7PV9)40, SLC4A4 (PDB: 6CAA)41, SLC1A5 (PDB: 5LMM)42, SLC7A5 (PDB: 6IRS)43, SLC29A1 (PDB: 6OB6)44. The superposed structures are performed for: SLC2A1Crystal versus SLC2A1QTY, SLC7A11CryoEM versus SLC7A11QTY, SLC4A4CryoEM versus SLC4A4QTY, SLC1A5Crystal versus SLC1A5QTY, SLC7A5CryoEM versus SLC7A5QTY and SLC29A1Crystal versus SLC29A1QTY (Tables 2 and 3).
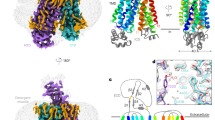
The experimentally-determined native structures and their in-silico-determined water-soluble QTY variants superposed within a few Å. Their RMSDs are as follows: SLC2A1 versus SLC2A1QTY (2.281 Å); for SLC7A11 versus SLC7A11QTY (0.933 Å); SLC4A4 versus SLC4A4QTY (0.445 Å); SLC1A5 versus SLC1A5QTY(0.965 Å); SLC7A5 versus SLC7A5QTY (1.815 Å); and SLC29A1 versus SLC29A1QTY (1.512 Å). (Fig. 3, Table 2). It can be seen from Fig. 3, these molecular structures, experimentally-determined and AlphaFold2-predicted visibly superpose very well. These results show that despite > 45% QTY substitutions in the transmembrane alpha-helices in the water-soluble QTY variants, their structures share rather similar 3-dimentional folds. These closely superposed structures perhaps confirm that the AlphaFold2’s predictions are highly accurate, since the predicted native structures are directly superposed with the experimentally determined X-ray crystal structures. Our AlphaFold2 predicted structures show the significant structural similarity between the native SLC transporter and their water-soluble QTY variants.
Superposed 6 solute carrier transporter crystal or CryoEM structures with QTY variants predicted by AlphaFold2. The X-ray crystal or CryoEM structures of the native transporters are obtained from the Protein Data Bank (PDB). The crystal or CryoEM structures (magenta) are superposed with QTY variants (cyan) predicted by AlphaFold2. The RMSD (Å) for each structure is in Table 2. (a) SLC2A1Crystal versus SLC2A1QTY (2.281 Å), (b) SLC7A11CryoEM versus SLC7A11QTY (0.933 Å), (c) SLC4A4CryoEM versus SLC4A4QTY (0.445 Å), (d) SLC1A5Crystal versus SLC1A5QTY (0.965 Å), (e) SLC7A5CryoEM versus SLC7A5QTY (1.815 Å), (f) SLC29A1Crystal versus SLC29A1QTY (1.512 Å). These superposed structures display that the crystal or CryoEM structures and their QTY variants have very similar molecular structures (Tables 2, 3). For clarity of direct comparisons, the N-terminus and C-terminus are deleted.
Because the X-ray crystal or CryoEM structures of the other 7 native SLCxxxx are not yet available, including SLC39A4, SLC39A6, SLC39A3, SLC9A1, SLC6A14, SLC4A7, SLC5A8 and SLC41A1, AlphaFold2 tool is used for the structural predictions. The RMSD in Å (residue mean-square distances) for these superposed structures are displayed (Fig. 4, Table 2). The examples are: SLC39A6 versus SLC39A6QTY (0.931 Å); SLC39A3 versus SLC39A3QTY (1.567 Å); SLC9A1 versus SLC9A1QTY (0.905 Å); SLC6A14 versus SLC6A14QTY (0.521 Å); SLC4A7 versus SLC4A7QTY (0.426 Å); SLC5A8 versus SLC5A8QTY (1.593 Å); and SLC41A1 versus SLC41A1QTY (1.914 Å) (Table 2). The AlphaFold2-predicted structures of both natural SLCs and their water-soluble SLC variants superpose very well implying that they share comparable structures despite the significant substitutions in the transmembrane alpha-helices (45–54%).
Superposed 7 native solute carrier transporters and their QTY variants that were predicted by AlphaFold2. The native structures (green) and their water-soluble QTY variants (cyan). For the superposed structures, the RMSD is in Å ( ). (a) SLC39A6 versus SLC39A6QTY (0.931 Å) (b) SLC39A3 versus SLC39A3QTY (1.567 Å), (c) SLC9A1 versus SLC9A1QTY (0.905 Å), (d) SLC6A14 versus SLC6A14QTY (0.521 Å), (e) SLC4A7 versus SLC4A7QTY (0.426 Å), (f) SLC5A8 versus SLC5A8QTY (1.593 Å), (g) SLC41A1 versus SLC41A1QTY (1.914 Å). The large N- and C-termini are removed for clarity. Please see Tables 2 and 3. For clarity, N-terminus, C-terminus and large loops are deleted.
Analysis of the hydrophobic surface of native transporters and the water-soluble QTY variants
It is known that the natural SLCxxxx have high hydrophobicity content, especially in the transmembrane alpha-helical segments. They are inherently insoluble in water and require surfactants to solubilize and stabilize them. These natural transporters quickly self-associate to form unstructured aggregation, precipitation, and no longer biologically functional without the highly selected surfactants.
In the natural SLCxxxx, the 6TM-12TM alpha-helices are directly embedded in the hydrophobic lipid bilayer. The hydrophobic side chains of phenylalanine, isoleucine, leucine, and valine interact with the hydrophobic lipid bilayers. Thus, the 6TM-12TM alpha-helices exhibit water-repelling hydrophobic surfaces (Figs. 5, 6).
Hydrophobic surface of 6 crystal structures of solute carrier transporters and the designed QTY variants. The native solute carrier transporters have many hydrophobic residues L, I, V and F in the transmembrane helices. After Q, T, and Y replacement of the L, I, V, F, the surfaces are much more hydrophilic. The hydrophobic surface (brownish) of the native transporters become more cyan color: (a) SLC2A1 versus SLC2A1QTY, (b) SLC7A11 versus SLC7A11QTY, (c) SLC4A4 versus SLC4A4QTY, (d) SLC1A5 versus SLC1A5QTY, (e) SLC7A5 versus SLC7A5QTY, (f) SLC29A1 versus SLC29A1QTY. The hydrophobic surface is largely reduced on the transmembrane helices for the QTY variants. These QTY variants converted to water-soluble form. For clarity, the N-terminus and C-terminus are deleted.
Hydrophobic surface of 8 AlphaFold2 predicted native solute carrier transporters and their QTY variants. The pairwise of AlphaFold2 predicted native transporters with hydrophobic surface (brownish color), and the QTY variant transporters (cyan color). (a) SLC39A6 versus SLC39A6QTY, (b) SLC39A3 versus SLC39A3QTY, (c) SLC9A1 versus SLC9A1QTY, (d) SLC6A14 versus SLC6A14QTY, (e) SLC4A7 versus SLC4A7QTY, (f) SLC5A8 versus SLC5A8QTY, (g) SLC41A1 versus SLC41A1QTY. For clarity, the large N- terminus and C-terminus are deleted.
After QTY substitutions of hydrophobic amino acids L, I, V, F, with hydrophilic amino acids Q, T and Y, the hydrophobic surfaces are decreased (Figs. 5, 6). The QTY changes hydrophobic 6TM-12TM into hydrophilic 6TM-12TM, without, however, significantly changing the alpha-helical molecular structures, as shown in Fig. 3. Analogous reproducible experimental results reported for chemokine and cytokine receptors in our previous publications31,32,33,34,35. However, our experimental results showed that the structure integrity, stability, and ligand-binding activities have been retained from the water-soluble QTY-variant chemokine receptors and cytokine receptors31,32,33,34,35.
There are three chemically distinct alpha-helix types. Type I: the water-soluble hydrophilic alpha-helix, commonly water-soluble enzymes in the cellular cytosols and extracellular circulating proteins including antibodies, protein and peptide hormones and more; Type II: the water-insoluble hydrophobic alpha-helix commonly in transmembrane proteins including hormone receptors, transporters, ion channels, G protein-coupled receptors, photosynthesis systems; and Type III: the amphiphilic alpha-helix, like a Janus that have a hydrophobic face on one side and a hydrophilic face on the other side. These three chemically distinct alpha-helical types have very similar structures, regardless their hydrophobicity and hydrophilicity45,46,47,48,49. This is the molecular foundation of the QTY code.
AlphaFold2 predictions
For over 65 years, scientists have made great efforts to predict protein folding. It was the dream of structural biologists and protein scientists to predict protein folding rapidly and accurately. With the advent of AlphaFold2 through machine learning, the tool is now available to predict protein structure by all scientists, almost free of charge. We can now study previously unattainable protein structures, particularly membrane-embedded transmembrane proteins.
Systematic bioinformatic studies revealed that in most organisms, ~ 20–30% genes code for membrane proteins50. It is known that the human genome codes for ~ 24% membrane proteins51. But structural determination of a single transmembrane protein is an extremely difficult process, traditionally requiring even decades of endeavor. There are many barriers, from gene expression, protein production, detergent selection, purification, detergent exchange, to maintaining their long-term stability and integrity as well as maintaining functionality to avoid irreversible aggregation. The numbers of integral transmembrane protein structures lag far behind water-soluble proteins. Recently, several groups have systematically analyzed the known structures and at least 16 different folds of ~ 400 members, 65 families of the solute carrier transporter52,53. These studies further provide insights into molecular structures and functions of these transporters.
Applying AlphaFold2 accurate protein structure predictions, we can directly compare the native structure with an AlphaFold2 predicted water-soluble QTY variant. With the QTY variant, expressed in various cells, it becomes possible to overcome the high barriers of studying membrane embedded transmembrane proteins.
We previously reported36,37 use of the AlphaFold2 tool to predict structures of the water-soluble variants of G protein coupled receptor chemokine receptors and glucose transporters, and compared them to the known experimentally-determined crystal or CryoEM structures.
One of the questions concerns what would be the utility of these water-soluble transporters that can no longer transport molecules cross the membrane since the water-soluble QTY variants can no longer insert themselves into the lipid bilayer membrane. It is plausible that after these transporters are rendered water-soluble, they can be used: (1) as water-soluble antigens to generate useful monoclonal antibodies in animals since they have many extracellular loops including few large loops, and (2) these anti-SLCxxx antibodies could be useful as research reagents for an assay system to study the transporters in tissue cell cultures and in vivo. These specific monoclonal antibodies can perhaps also be useful (a) as therapeutics to treat diseases including pancreatic cancer, (b) as diagnostic reagent to monitor cancer treatments and perhaps (c) for early pancreatic cancer detection.
Our current study using the AlphaFold 2 demonstrates that the water-soluble QTY-variant structures of SLC transporters are substantially similar to the native structures. AlphaFold 2 is a very useful approach to predict other membrane embedded transmembrane proteins. QTY is a useful approach for working with difficult-to-study hydrophobic proteins. The SLC transporter water-soluble QTY variants not only could be used for designs of molecular machines, but also as water-soluble antigens for generating therapeutic monoclonal antibodies and for accelerating drug discovery.
Methods
Protein sequence alignments and other characteristics
The native protein sequences for SLC transporters and their QTY-variant sequences are aligned using the same methods previously described33,34. The website Expasy (https://web.expasy.org/compute_pi/) was used to calculate the molecular weights (MW) and pI values of the proteins.
AlphaFold2 predictions
AlphaFold228,30 Program https://github.com/sokrypton/ColabFold was used for the structure predictions of the QTY variants following the instructions at the website on 2 × 20 Intel Xeon Gold 6248 cores, 384 GB RAM, and a Nvidia Volta V100 GPU. The European Bioinformatics Institute (EBI, https://alphafold.ebi.ac.uk) has all AlphaFold2-predicted structures. The Uniprot website https://www.uniprot.org has each protein ID, entry name, description, and FASTA sequence. The data was taken from UniProt using a custom Python code. The QTY method website (https://pss.sjtu.edu.cn/) can convert the FASTA protein sequences into their water-soluble versions. These steps were optimized using Python libraries for web applications such as requests and splinter.
Superposed structures
The molecular structures are taken from PDB https://www.rcsb.org. They include SLC2A1 (PDB: 6THA)39, SLC7A11 (PDB: 7P9U)40, SLC4A4 (PDB: 6CAA)41, SLC1A5 (PDB: 5LMM)42, SLC7A5 (PDB: 6IRS)43, SLC29A1 (PDB: 6OB6)44. AlphaFold2 predictions of 8 native SLC transporters and their QTY variants were carried out using the AlphaFold2 program at https://github.com/sokrypton/ColabFold. Uniprot https://www.uniprot.org is the source for all 13 SLC transporter protein sequences and AlphaFold2 was performed to predict QTY variant structures. These structures are superposed using PyMOL https://pymol.org/2/.
Structure visualization
Two key programs were used for structure visualization: PyMOL https://pymol.org/2/ and UCSF Chimera https://www.rbvi.ucsf.edu/chimera/. PyMOL program is used for the superposed models, while hydrophobicity models were rendered using Chimera.
Ethical approval
(1) All methods were carried out in accordance with relevant guidelines and regulations. (2) All experimental protocols were approved by a named institutional and licensing committee. (3) Neither human biological samples, nor human subjects were used in the study. This is a completely digital structural biology study using the publicly available AlphaFold2 machine learning program.
Data availability
European Bioinformatics Institute (EBI, https://alphafold.ebi.ac.uk) is the deposit site for all AlphaFold2 predicted > 214 million protein structures. Please use the website for more detailed information, please contact the first author Eva Smorodin at ribes.ev@gmail.com and at website: https://github.com/eva-smorodina/slc for the QTY code designed water-soluble SLC variants.
References
Wu, Z. et al. Emerging roles of the solute carrier family in pancreatic cancer. Clin. Transl. Med. 11, e356 (2021).
Pizzagalli, M. D., Bensimon, A. & Superti-Furga, G. A guide to plasma membrane solute carrier proteins. FEBS J. 288, 2784–2835 (2021).
Sutherland, R., Meeson, A. & Lowes, S. Solute transporters and malignancy: Establishing the role of uptake transporters in breast cancer and breast cancer metastasis. Cancer Metastasis Rev. 39, 919–932 (2020).
Schumann, T. et al. Solute carrier transporters as potential targets for the treatment of metabolic disease. Pharmacol. Rev. 72, 343–379 (2020).
Höglund, P. J., Nordström, K. J., Schiöth, H. B. & Fredriksson, R. The solute carrier families have a remarkably long evolutionary history with the majority of the human families present before divergence of bilaterian species. Mol. Biol. Evol. 28, 1531–1541 (2011).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: The next generation. Cell 144, 646–674 (2011).
Ito, H. et al. Glucose transporter-1 gene expression is associated with pancreatic cancer invasiveness and MMP-2 activity. Surgery 136, 548–556 (2004).
Nagarajan, A. et al. Paraoxonase 2 facilitates pancreatic cancer growth and metastasis by stimulating GLUT1-mediated glucose transport. Mol. Cell 67, 685-701.e6 (2017).
Sharen, G. et al. Prognostic value of GLUT-1 expression in pancreatic cancer: results from 538 patients. Oncotarget 8, 19760–19767 (2017).
Zhu, J. H. MiR-139-5p/SLC7A11 inhibits the proliferation, invasion and metastasis of pancreatic carcinoma via PI3K/Akt signaling pathway. Biochim. Biophys. Acta Mol. Basis Dis. 1866, 165747 (2020).
Zhang, X. et al. Hsa circRNA 001587 upregulates SLC4A4 expression to inhibit migration, invasion and angiogenesis of pancreatic cancer cells via binding to microRNA-223. Am. J. Physiol. Gastrointest. Liver Physiol. 319, G703–G717 (2020).
Yoo, H. C. et al. A variant of SLC1A5 is a mitochondrial glutamine transporter for metabolic reprogramming in cancer cells. Cell Metab. 31, 267–283 (2020).
Kaira, K. et al. Prognostic significance of L-type amino-acid transporter 1 expression in surgically resected pancreatic cancer. Br. J. Cancer 107, 632–638 (2012).
Altan, B. et al. Relationship between LAT1 expression and resistance to chemotherapy in pancreatic ductal adenocarcinoma. Cancer Chemother. Pharmacol. 81, 141–153 (2018).
Unno, J. et al. LIV-1 enhances the aggressive phenotype through the induction of epithelial to mesenchymal transition in human pancreatic carcinoma cells. Int. J. Oncol. 35, 813–821 (2009).
Wan, Z. & Wang, X. Role of SLC39A6 in the development and progression of liver cancer. Oncol. Lett. 23, e77 (2022).
Costello, L. C. et al. Decreased zinc and downregulation of ZIP3 zinc uptake transporter in the development of pancreatic adenocarcinoma. Cancer Biol. Ther. 12, 297–303 (2011).
Franklin, R. B. et al. The cytotoxic role of RREB1, ZIP3 zinc transporter, and zinc in human pancreatic adenocarcinoma. Cancer Biol. Ther. 15, 1431–1437 (2014).
Takagishi, T. et al. Recent advances in the role of SLC39A/ZIP zinc transporters in vivo. Int. J. Mol. Sci. 18, 2708 (2017).
Olszewski, U. & Hamilton, G. Neurotensin signaling induces intracellular alkalinization and interleukin-8 expression in human pancreatic cancer cells. Mol. Oncol. 3, 204–213 (2009).
Cardone, R. A. et al. A novel NHE1-centered signaling cassette drives epidermal growth factor receptor-dependent pancreatic tumor metastasis and is a target for combination therapy. Neoplasia 17, 155–166 (2015).
Malinda, R. R. et al. TGFβ signaling increases net acid extrusion, proliferation and invasion in Panc-1 pancreatic cancer cells: SMAD4 dependence and link toMerlin/NF2 signaling. Front. Oncol. 10, 687 (2020).
Coothankandaswamy, V. et al. Amino acid transporter SLC6A14 is a novel and effective drug target for pancreatic cancer. Br. J. Pharmacol. 173, 3292–3306 (2016).
Ramirez, C. et al. Plasma membrane V-ATPase controls oncogenic RAS-induced macropinocytosis. Nature 576, 477–481 (2019).
Park, J. Y. et al. Silencing of the candidate tumor suppressor gene solute carrier family 5 member 8 (SLC5A8) in human pancreatic cancer. Pancreas 36, e32–e39 (2008).
Helm, J. et al. SLC5A8 nuclear translocation and loss of expression are associated with poor outcome in pancreatic ductal adenocarcinoma. Pancreas 41, 904–909 (2012).
Xie, J. et al. Magnesium transporter protein solute carrier family 41 member 1 suppresses human pancreatic ductal adenocarcinoma through magnesium-dependent Akt/mTOR inhibition and bax-associated mitochondrial apoptosis. Aging 11, 2681–2698 (2019).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Baek, M. et al. Accurate prediction of protein structures and interactions using a three-track neural network. Science 373, 871–876 (2021).
Varadi, M. et al. AlphaFold protein structure database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 50(D1), D439–D444 (2021).
Zhang, S. et al. QTY code enables design of detergent-free chemokine receptors that retain ligand-binding activities. Proc. Natl. Acad. Sci. U. S. A. 115, E8652–E8659 (2018).
Qing, R. et al. QTY code designed thermostable and water-soluble chimeric chemokine receptors with tunable ligand-binding activities. Proc. Natl. Acad. Sci. U. S. A. 116, 25668–25676 (2019).
Tegler, L. T. et al. G protein-coupled receptor CXCR4 designed by the QTY code becomes more hydrophilic and retains cell-signaling activity. Sci. Rep. 10, 21371 (2020).
Hao, S. L. et al. QTY code-designed water-soluble Fc-fusion cytokine receptors bind to their respective ligands. QRB Discov. 1, e4 (2020).
Qing, R. et al. Non-full-length water-soluble CXCR4QTY, CCR5QTY chemokine receptors and implication for overlooked truncated membrane receptors. iScience 23, 101670 (2020).
Skuhersky, M. et al. Comparing native crystal structures and AlphaFold2 predicted water-soluble G protein-coupled receptor QTY variants. Life 11, 1285 (2021).
Smorodina, E. et al. Comparing 2 crystal structures and 12 AlphaFold2 predicted human membrane glucose transporters and their water-soluble QTY variants. QRB Discov. 3, e5. https://doi.org/10.1017/qrd.2022.6 (2022).
Tao, F. et al. Enabling QTY server for designing water-soluble α-helical transmembrane proteins. MBio 13, e03604-e3621 (2022).
Deng, D. et al. Molecular basis of ligand recognition and transport by glucose transporters. Nature 526, 391–396 (2015).
Parker, J. L. et al. Molecular basis for redox control by the human cystine/glutamate antiporter system xc. Nat. Commun. 12, 7147. https://doi.org/10.1038/s41467-021-27414-1 (2021).
Huynh, K. W. et al. CryoEM structure of the human SLC4A4 sodium-coupled acid-base transporter NBCe1. Nat. Commun. 9, 900. https://doi.org/10.1038/s41467-018-03271-3 (2018).
Canul-Tec, J. C. et al. Structure and allosteric inhibition of excitatory amino acid transporter 1. Nature 544, 446–451 (2017).
Yan, R. et al. Structure of the human LAT1-4F2hc heteromeric amino acid transporter complex. Nature 568, 127–130 (2019).
Wright, N. J. & Lee, S. Y. Structures of human ENT1 in complex with adenosine reuptake inhibitors. Nat. Struct. Mol. Biol. 26, 599–606 (2019).
Pauling, L., Corey, R. B. & Branson, H. R. The structure of proteins: Two hydrogen-bonded helical configurations of the polypeptide chain. Proc. Natl Acad. Sci. U. S. A. 37, 205–211 (1951).
Brändén, C. I. & Tooze, J. Introduction to Protein Structure 1st edn. (Garland Science, USA, 1991).
Fersht, A. Structure and Mechanism in Protein Science: A Guide to Enzyme Catalysis and Protein Folding 2nd edn. (Wolrd Scientific Publishing, 2017).
Liljas, A. et al. Textbook of Structural Biology 2nd edn. (World Scientific Publishing Company, 2017).
Zhang, S. & Egli, M. Hiding in plain sight: Three chemically distinct alpha-helix types. Q. Rev. Biophys. 55, e7. https://doi.org/10.1017/S0033583522000063 (2022).
Krogh, A., Larsson, B., von Heijne, G. & Sonnhammer, E. L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 305, 567–580 (2001).
Fagerberg, L., Jonasson, K., von Heijne, G., Uhlén, M. & Berglund, L. Prediction of the human membrane proteome. Proteomics 10, 1141–1149 (2010).
Bai, X., Moraes, T. F. & Reithmeier, R. A. F. Structural biology of solute carrier (SLC) membrane transport proteins. Mol. Membr. Biol. 34, 1–32 (2017).
Xie, T. et al. Rational exploration of fold atlas for human solute carrier proteins. Structure 30, 1321-1330.e5 (2022).
Acknowledgements
We sincerely thank Dr. Arthur Zalevsky of University of California at San Francisco to provide valuable suggestions and discussion for several CryoEM structures of SLC7A11 and to point out that the CryoEM structure 7CCS is a designed thermal stable variant with 101 mutations (20.2%) in this SLC7A11, but it retained the similar CryoEM structure. The This work was primarily supported by PT Metiska Farma. We thank Dorrie Langsley for English editing.
Funding
This work is primarily supported by PT Metiska Farma, Jakarta, Indonesia.
Author information
Authors and Affiliations
Contributions
Conceptualization, S.Z.; methodology, E.S., I.D., F.T.; software, AlphaFold2, E.S., I.D.; validation, E.S., I.D.; formal analysis, E.S., I.D., F.T.; investigation, F.T., Q.R., S.Y.; resources, S.Y, data curation, E.S., I.D.; writing of the original draft preparation, S.Z.; writing, reviewing and editing, E.S., I.D., F.T., Q.R., S.Y., S.Z.; visualization, E.S., I.D.; supervision, S.Z.; project administration, S.Z.; funding acquisition, S.Y. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Massachusetts Institute of Technology (MIT) filed several patent applications for the QTY code and OH2Laboratories licensed the technology specifically for G protein-coupled receptors from MIT. Shuguang Zhang is an inventor and has a minor equity of OH2Laboratories stock shares. MIT and Avalon GloboCare jointly filed a patent application for glucose transporters but did not file any patent application on the solute carrier transporters. After submitting the initial manuscript, Shuguang Zhang formed a startup 511 Therapeutics, Inc. in August 2022 to generate therapeutic monoclonal antibodies and holds majority equity. The PT Metiska Farma sponsored the study but had no influence and interference in the design of the study, in the data collection, analyses, or interpretation of data and in the writing of the manuscript, or in the decision to publish the results. All other authors have no competing interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Smorodina, E., Diankin, I., Tao, F. et al. Structural informatic study of determined and AlphaFold2 predicted molecular structures of 13 human solute carrier transporters and their water-soluble QTY variants. Sci Rep 12, 20103 (2022). https://doi.org/10.1038/s41598-022-23764-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-23764-y
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.