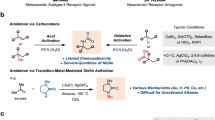
Abstract
Electrophilic halogenation is a widely used tool employed by medicinal chemists to either pre-functionalize molecules for further diversity or incorporate a halogen atom into drugs or drug-like compounds to solve metabolic problems or modulate off-target effects. Current methods to increase the power of halogenation rely on either the invention of new reagents or activating commercially available reagents with various additives such as Lewis or Brønsted acids, Lewis bases and hydrogen-bonding activators. There is a high demand for new reagents that can halogenate otherwise unreactive compounds under mild conditions. Here we report the invention of a class of halogenating reagents based on anomeric amides, taking advantage of the energy stored in the pyramidalized nitrogen of N–X anomeric amides as a driving force. These robust halogenating methods are compatible with a variety of functional groups and heterocycles, as exemplified on over 50 compounds (including 13 gram-scale examples and 1 flow chemistry scale-up).

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre under deposition numbers CCDC 2270492 (5), 2238041 (6), 2252734 (7), 2247860 (16-Cl), 2287564 (28-Cl), 2252735 (34-Cl), 2263724 (38-Cl), 2255203 (39-Cl), 2287572 (3-Br), 2263723 (23-Br), 2257302 (25-Br), 2261009 (38-Br), 2278375 (39-Br) and 2261010 (43-Br). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Campeau, L.-C. & Hazari, N. Cross-coupling and related reactions: connecting past success to the development of new reactions for the future. Organometallics 38, 3–35 (2019).
Smith, B. R., Eastman, C. M. & Njardarson, J. T. Beyond C, H, O, and N! Analysis of the elemental composition of U.S. FDA approved drug architectures. J. Med. Chem. 57, 9764–9773 (2014).
Hernandes, M. Z., Cavalcanti, S. M., Moreira, D. R., de Azevedo Junior, W. F. & Leite, A. C. Halogen atoms in the modern medicinal chemistry: hints for the drug design. Curr. Drug Targets 11, 303–314 (2010).
Chiodi, D. & Ishihara, Y. “Magic chloro”: profound effects of the chlorine atom in drug discovery. J. Med. Chem. 66, 5305–5331 (2023).
Curtin, M. L. et al. Diaminopyrimidines as dual inhibitors of KDR and Aurora B kinases. Bioorg. Med. Chem. Lett. 22, 4750–4755 (2012).
Chong, P. et al. Rational design of potent non-nucleoside inhibitors of HIV-1 reverse transcriptase. J. Med. Chem. 55, 10601–10619 (2012).
Taylor, R. Electrophilic Aromatic Substitution (Wiley, 1990).
Guillemard, L., Kaplaneris, N., Ackermann, L. & Johansson, M. J. Late-stage C–H functionalization offers new opportunities in drug discovery. Nat. Rev. Chem. 5, 522–545 (2021).
Cernak, T., Dykstra, K. D., Tyagarajan, S., Vachal, P. & Krska, S. W. The medicinal chemist’s toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev. 45, 546–576 (2016).
Moir, M., Danon, J. J., Reekie, T. A. & Kassiou, M. An overview of late-stage functionalization in today’s drug discovery. Expert Opin. Drug Discov. 14, 1137–1149 (2019).
Börgel, J. & Ritter, T. Late-stage functionalization. Chem 6, 1877–1887 (2020).
Gutekunst, W. R. & Baran, P. S. C–H functionalization logic in total synthesis. Chem. Soc. Rev. 40, 1976–1991 (2011).
Yamaguchi, J., Yamaguchi, A. D. & Itami, K. C–H bond functionalization: emerging synthetic tools for natural products and pharmaceuticals. Angew. Chem. Int. Ed. 51, 8960–9009 (2012).
Brown, D. G. & Boström, J. Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone? J. Med. Chem. 59, 4443–4458 (2016).
Roughley, S. D. & Jordan, A. M. The medicinal chemist’s toolbox: an analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 54, 3451–3479 (2011).
Chen, X., Hao, X.-S., Goodhue, C. E. & Yu, J.-Q. Cu(II)-catalyzed functionalizations of aryl C–H bonds using O2 as an oxidant. J. Am. Chem. Soc. 128, 6790–6791 (2006).
Das, R. & Kapur, M. Transition-metal-catalyzed site-selective C–H halogenation reactions. Asian J. Org. Chem. 7, 1524–1541 (2018).
Murphy, J. M., Liao, X. & Hartwig, J. F. Meta halogenation of 1,3-disubstituted arenes via iridium-catalyzed arene borylation. J. Am. Chem. Soc. 129, 15434–15435 (2007).
Boyle, B. T., Levy, J. N., de Lescure, L., Paton, R. S. & McNally, A. Halogenation of the 3-position of pyridines through Zincke imine intermediates. Science 378, 773–779 (2022).
Cao, H., Cheng, Q. & Studer, A. Radical and ionic meta-C–H functionalization of pyridines, quinolines, and isoquinolines. Science 378, 779–785 (2022).
Ni, S. et al. Nickel meets aryl thianthrenium salts: Ni(I)-catalyzed halogenation of arenes. J. Am. Chem. Soc. 145, 9988–9993 (2023).
Latham, J., Brandenburger, E., Shepherd, S. A., Menon, B. R. K. & Micklefield, J. Development of halogenase enzymes for use in synthesis. Chem. Rev. 118, 232–269 (2018).
Höfler, G. T., But, A. & Hollmann, F. Haloperoxidases as catalysts in organic synthesis. Org. Biomol. Chem. 17, 9267–9274 (2019).
Crowe, C. et al. Halogenases: a palette of emerging opportunities for synthetic biology–synthetic chemistry and C–H functionalisation. Chem. Soc. Rev. 50, 9443–9481 (2021).
Attanasi, O. A. et al. Tetrabromo hydrogenated cardanol: efficient and renewable brominating agent. Org. Lett. 8, 4291–4293 (2006).
Rodriguez, R. A. et al. Palau’chlor: a practical and reactive chlorinating reagent. J. Am. Chem. Soc. 136, 6908–6911 (2014).
Lu, Z. et al. CFBSA: a novel and practical chlorinating reagent. Chem. Commun. 51, 14852–14855 (2015).
Zhang, Y., Shibatomi, K. & Yamamoto, H. Lewis acid catalyzed highly selective halogenation of aromatic compounds. Synlett 2005, 2837–2842 (2005).
Mo, F. et al. Gold-catalyzed halogenation of aromatics by N-halosuccinimides. Angew. Chem. Int. Ed. 49, 2028–2032 (2010).
Wang, W. et al. Catalytic electrophilic halogenation of arenes with electron-withdrawing substituents. J. Am. Chem. Soc. 144, 13415–13425 (2022).
Maddox, S. M., Nalbandian, C. J., Smith, D. E. & Gustafson, J. L. A practical Lewis base catalyzed electrophilic chlorination of arenes and heterocycles. Org. Lett. 17, 1042–1045 (2015).
Samanta, R. C. & Yamamoto, H. Selective halogenation using an aniline catalyst. Chem. Eur. J. 21, 11976–11979 (2015).
Song, S. et al. DMSO-catalysed late-stage chlorination of (hetero)arenes. Nat. Catal. 3, 107–115 (2020).
Gerdes, R. G., Glover, S. A., ten Have, J. F. & Rowbottom, C. A. N-acetoxy-N-alkoxyamides – a new class of nitrenium ion precursors which are mutagenic. Tetrahedron Lett. 30, 2649–2652 (1989).
Glover, S. A. Anomeric amides—structure, properties and reactivity. Tetrahedron 54, 7229–7271 (1998).
Kennedy, S. H., Dherange, B. D., Berger, K. J. & Levin, M. D. Skeletal editing through direct nitrogen deletion of secondary amines. Nature 593, 223–227 (2021).
Qi, T. et al. Iron(II)-catalyzed nitrene transfer reaction of sulfoxides with N-acyloxyamides. Org. Lett. 24, 5674–5678 (2022).
Sutton, A. D., Williamson, M., Weismiller, H. & Toscano, J. P. Optimization of HNO production from N,O-bis-acylated hydroxylamine derivatives. Org. Lett. 14, 472–475 (2012).
Beebe, T. R. & Wolfe, J. W. N-bromination of amides, imides, and sulfonamides with acetyl hypobromite. J. Org. Chem. 35, 2056–2057 (1970).
Pitzer, L., Schäfers, F. & Glorius, F. Rapid assessment of the reaction-condition-based sensitivity of chemical transformations. Angew. Chem. Int. Ed. 58, 8572–8576 (2019).
Kruszyk, M., Jessing, M., Kristensen, J. L. & Jørgensen, M. Computational methods to predict the regioselectivity of electrophilic aromatic substitution reactions of heteroaromatic systems. J. Org. Chem. 81, 5128–5134 (2016).
Korganow, A.-S., Eftekhari, P., Wagner, A. & Baehr, C. Analogues of hydroxychloroquine (HCQ) without retinal toxicity. US patent 201,906,227,878 A1 (2019).
Regnier, G., Canevari, R. J., Laubie, M. J. & Le Douarec, J. C. Synthesis and vasodilator activity of new piperazine derivatives. J. Med. Chem. 11, 1151–1155 (1968).
Rohrig, S. Substituted oxazolidinones and the use thereof. US patent 20100184767 A1 (2010).
Esumi, N., Suzuki, K., Nishimoto, Y. & Yasuda, M. Generation of α-iminyl radicals from α-bromo cyclic N-sulfonylimines and application to coupling with various radical acceptors using a photoredox catalyst. Chem. Eur. J. 24, 312–316 (2018).
Kim, C.-S. et al. Determination of the absolute configuration of (+)-2,7(14),10-bisabolatrien-1-ol-4-one from Japanese cedar, Cryptomeria japonica. Biosci. Biotechnol. Biochem. 66, 1997–2000 (2002).
Kobayashi, K. et al. Total synthesis of highly oxygenated bisabolane sesquiterpene isolated from Ligularia lankongensis: relative and absolute configurations of the natural product. J. Org. Chem. 83, 703–715 (2018).
Bodman, G. T. & Chervin, S. Use of ARC in screening for explosive properties. J. Hazard. Mater. 115, 101–105 (2004).
Bilke, M., Losch, P., Vozniuk, O., Bodach, A. & Schüth, F. Methane to chloromethane by mechanochemical activation: a selective radical pathway. J. Am. Chem. Soc. 141, 11212–11218 (2019).
Brown, R. N. The crystal structure of N-chlorosuccinimide. Acta Cryst. 14, 711–715 (1961).
Beatty, J. W. et al. Discovery of potent and selective non-nucleotide small molecule inhibitors of CD73. J. Med. Chem. 63, 3935–3955 (2020).
Baloniak, S. & Katresiak, A. Synthesis and reactivity of some 1,2,4-triazolo[4,3-b]pyridazine derivatives. Pol. J. Chem. 68, 683–691 (1994).
Chen, C.-M., Chen, J.-X. & To, C. T. Solvent-free mechanochemical chlorination of pyrazoles with trichloroisocyanuric acid. Green Chem. 25, 2559–2562 (2023).
Krake, E. F. & Baumann, W. Selective oxidation of clopidogrel by peroxymonosulfate (PMS) and sodium halide (NaX) system: an NMR study. Molecules 26, 5921–5932 (2021).
Acknowledgements
Financial support for this work was provided by the National Institutes of Health (grant no. GM-118176, P.S.B.). We thank D.-H. Huang and L. Pasternack (Scripps Research) for assistance with NMR spectroscopy; M. A. Schmidt (BMS) for insightful discussion on computational studies; M. Gembicky and E. Samolova (UCSD) for X-ray crystallographic analysis; and J. Chen, B. Sanchez and Q. N. Wong (Scripps Research ASF) for high-resolution mass spectrometry.
Author information
Authors and Affiliations
Contributions
Y.W., C.B., Y.K., L.N.G. and P.S.B. conceptualized the study. Y.W. and C.B. developed the reagents and conducted reaction optimization. Y.W., C.B., M.D.P., M.R.C., M.S.O., C.C.T., D.C., E.A.L. and J.X.Q. contributed to the substrate scope and analysed the data. K.C.H. and A.V. designed and performed flow chemistry scale-up. L.N.G. performed the DFT calculations. L.S., P.F.R. and S.Z. performed the safety experiments. J.B.B. performed the X-ray crystallographic analysis. Y.W., C.B., Y.K., L.N.G., L.S., K.C.H. and P.S.B. wrote the paper. P.S.B. supervised this work. All authors contributed to discussions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–23, Schemes 1–3, experimental procedures, product characterization and NMR spectra.
Supplementary Data 1
Computational data.
Supplementary Data 2
Crystallographic data for compound 3-Br (CCDC reference 2287572).
Supplementary Data 3
Crystallographic data for compound 5 (CCDC reference 2270492).
Supplementary Data 4
Crystallographic data for compound 6 (CCDC reference 2238041).
Supplementary Data 5
Crystallographic data for compound 7 (CCDC reference 2252734).
Supplementary Data 6
Crystallographic data for compound 16-Cl (CCDC reference 2247860).
Supplementary Data 7
Crystallographic data for compound 23-Br (CCDC reference 2263723).
Supplementary Data 8
Crystallographic data for compound 25-Br (CCDC reference 2257302).
Supplementary Data 9
Crystallographic data for compound 28-Cl (CCDC reference 2287564).
Supplementary Data 10
Crystallographic data for compound 34-Cl (CCDC reference 2252735).
Supplementary Data 11
Crystallographic data for compound 38-Br (CCDC reference 2261009).
Supplementary Data 12
Crystallographic data for compound 38-Cl (CCDC reference 2263724).
Supplementary Data 13
Crystallographic data for compound 39-Br (CCDC reference 2278375).
Supplementary Data 14
Crystallographic data for compound 39-Cl (CCDC reference 2255203).
Supplementary Data 15
Crystallographic data for compound 43-Br (CCDC reference 2261010).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Bi, C., Kawamata, Y. et al. Discovery of N–X anomeric amides as electrophilic halogenation reagents. Nat. Chem. (2024). https://doi.org/10.1038/s41557-024-01539-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41557-024-01539-4