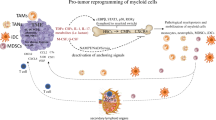
Abstract
Maturing immunometabolic research empowers immune regulation novel approaches. Progressive metabolic adaptation of tumor cells permits a thriving tumor microenvironment (TME) in which immune cells always lose the initial killing capacity, which remains an unsolved dilemma even with the development of immune checkpoint therapies. In recent years, many studies on tumor immunometabolism have been reported. The development of immunometabolism may facilitate anti-tumor immunotherapy from the recurrent crosstalk between metabolism and immunity. Here, we discuss clinical studies of the core signaling pathways of immunometabolism and their inhibitors or agonists, as well as the specific functions of these pathways in regulating immunity and metabolism, and discuss some of the identified immunometabolic checkpoints. Understanding the comprehensive advances in immunometabolism helps to revise the status quo of cancer treatment.

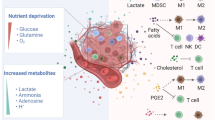
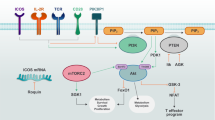
An overview of the new landscape of immunometabolism. The PI3K pathway promotes anabolism and inhibits catabolism. The LKB1 pathway inhibits anabolism and promotes catabolism. Overactivation of PI3K/AKT/mTOR pathway and IDO, IL4I1, ACAT, Sirt2, and MTHFD2 promote immunosuppression of TME formation, as evidenced by increased Treg and decreased T-cell proliferation. The LKBI-AMPK pathway promotes the differentiation of naive T cells to effector T cells and memory T cells and promotes anti-tumor immunity in DCs.
Similar content being viewed by others
Facts
-
Metabolic vulnerability of immune cells in the TME.
-
Targeting the metabolic pathways of immune cells is an effective way to modulate the immune response.
-
The development of immunometabolism may facilitate anti-tumor immunotherapy from the crosstalk between metabolism and immunity.
-
PI3K/AKT/mTOR and LKB1-AMPK are core signaling pathways in immunometabolism.
-
Immunometabolic checkpoints have great potential.
Open questions
-
How to reverse the metabolic vulnerability of immune cells in TME?
-
How can we use immunometabolic modulation to inhibit cancer cell growth?
-
How do the PI3K/AKT/mTOR and LKB1-AMPK pathways regulate immunometabolism?
-
What are the potential findings of immunometabolic checkpoints?
Introduction
Cancer is a serious global health problem. Although traditional therapies have prolonged many patients’ lives, the presence of stronger side effects has prompted researchers to work toward finding a method that can specifically destroy tumor cells without affecting normal cells [1, 2]. Understanding the multi-effector molecules of immunometabolism will confer more clinical research significance on tumor therapy [3]. Alternative metabolic pathways are regulated by tumor cells to adapt to specific nutrients. Cancer cells constantly exhibit metabolic reprogramming during proliferation which mediates immune escape as well as drug resistance [4]. Still, in contrast to tumor cells, immune cells may not inherently have a strong metabolic flexibility similar to that of tumor cells, which would result in an overall bias toward an immunosuppressive state in the TME, thus promoting the malignant process [5,6,7].
Tumors are not only aggregates of malignant cells but also well-organized complex ecosystems [8]. Cancer cells communicate directly or indirectly with the surrounding cellular microenvironment and evolve features to promote their survival [9,10,11]. The dilemma of tumor cells thrives while cytotoxic immune cells are hindered in the TME, which has been a challenge in cancer immunotherapy [12]. However, a recent discovery suggests that the metabolic dominance of tumor cells could be reprogrammed by cytotoxic immune cells, thereby reversing inequalities in the TME which benefits immune cells. Poznanski et al. showed that natural killer (NK) cells with Warburg metabolism and substrate flexibility not only maintained metabolic adaptability but also significantly enhanced tumor-killing capacity under unfavorable conditions of TME [13].
Following extensive research in cancer metabolism during the previous two decades, the latest progress in immunometabolism has further revealed promising signs of metabolic targets modulating anti-cancer immunity. The role of immune signaling networks in immunometabolism is a promising area of research with extensive impact on the therapy of cancer [3]. We focus on the key targets of each of these critical signaling pathways, the upstream and downstream signaling molecules and their mediated cell death as well as specific immune responses, and the clinical studies of small molecule inhibitors or activators of key targets. In addition, some immunometabolic checkpoints that have been reported to play important regulatory roles are also included. Therefore, this review provides essential information about molecular signaling in the field of immunometabolism and targeting strategies in oncology research, as well as highlights how this field drives advances in the therapy of human tumors.
Metabolic reprogramming in tumor cells
Studies have shown that metabolic reprogramming of tumor cells confers the potential for cancer cells to grow and proliferate in nutrient-deficient TME [14]. One such change in the metabolic pattern of tumor cells was first discovered in 1930 by the German biochemist Otto Warburg, which implied that most tumor cells do not generate energy by the conventional efficient tricarboxylic acid cycle, but instead supply themselves with energy through glycolysis, which is relatively inefficient in terms of energy production [15, 16]. Fast-growing malignant cells usually have a 200-fold higher glycolytic rate than the normal tissue, even in well-oxygenated environments [17]. Such reprogrammed cellular metabolism is now considered a hallmark of cancer [18, 19].
The heterogeneity of tumors and the high demand for nutrients give them a complex metabolic pattern. Besides relying heavily on glycolysis for energy, neoplastic cells promote self-proliferation using glutamine, serine, arginine, fatty acids, and lipids to maintain the harsh anabolic demands and energy productivity [20, 21].
Metabolic programs in immune cells
Adapted to different tissue environments, immune cells in TME develop specific metabolic characteristics [22, 23]. Different types of immune cells have specific nutritional requirements, using T-cell subsets as well-characterized examples for metabolic adaptation in TME [24]. Helper T cells and effector T cells conform to the “Warburg effect” by taking up large amounts of glucose and accelerating glycolysis while also increasing the rate of oxidative phosphorylation and consuming more glutamine [25,26,27]. In contrast to these two types of cells, regulatory T cells and memory T cells continue to derive most of the energy from the oxidative phosphorylation process, even after activation, when they preferentially consume fatty acids rather than amino acids and glucose [28, 29].
Metabolic dysfunction of T cells may result in a certain loss of immune function [30]. For instance, Inhibition of pyruvate dehydrogenase kinase, a positive regulator of aerobic glycolysis, disrupts the balance between Teffs and Tregs in the CD4+ T-cell subset, decreasing the inflammatory capacity of Th17 cells and promoting Treg cell production [31]. It has been suggested that regulation or reprogramming of metabolic alterations in tumor-infiltrating T cells may represent a potential strategy to revitalize dysfunctional T cells for cancer therapy [32]. In summary, the integration of the metabolic activity of T cells with the functional requirements of each T-cell lineage is an essential aspect of maintaining immune homeostasis and function.
Crosstalk between immune and metabolic signaling in TME
The constant crosstalk between immune and metabolic signals in TME allows immune and tumor cells with different metabolic patterns. The metabolism of tumor cells causes a large deficiency of nutritional substrates, including glucose and glutamine, in the TME, which results in abnormal metabolism and function of the immune cell population surrounding T cells [7, 33]. Based on the continuous crosstalk between immune and metabolic signals in TME and the effect of metabolic pathways in immune cells, integrating immunometabolic signaling pathways with phosphatidylinositol-3 kinase (PI3K)-protein kinase B (AKT/PKB), mechanistic target of rapamycin (mTOR) and liver kinase B1–5′ AMP-activated protein kinase (LKB1-AMPK) as the central link between immune signaling and metabolic pathways considerably influences tumor progression.
PI3K/Akt/mTOR signaling is one of the most critical intracellular signaling pathways controlling essential cellular functions, but key components of the pathway are frequently dysregulated in a variety of cancers [34]. For example, PI3K signaling is overactivated in breast cancer, inhibition of PI3K reduces the incidence of triple-negative and estrogen receptor-positive breast cancer, and in advanced and metastatic breast cancer, PI3KCA mutations may lead to chemoresistance and poor prognosis [35, 36]. AMPK, as the central metabolism that controls glucose and lipid metabolism, constitutes the most important signaling pathway with LKB1 in response to nutrient and intracellular energy changes. A study suggests that LKB1-AMPK axis inhibition determines esophageal squamous cell carcinoma cell fate from cellular senescence to glutamine-addicted survival [37]. With the deepening of immunometabolic studies, it has been shown that PI3K/Akt/mTOR and LKB1-AMPK, as core immunometabolic signaling pathways, can largely influence tumor progression in the continuous crosstalk between immune and metabolic signals [38].
PI3K-Akt signaling pathway
The PI3K/AKT/mTOR pathway tends to be overactivated by cancer, which is considered a promising therapeutic target [39]. PI3K is rapidly activated upon receiving an upstream signal stimulus, affecting a series of downstream targets, including AKT, mTOR, glycogen synthase kinase-3 (GSK3), ATP-citrate lyase (ACLY), etc., performing roles by increasing anabolism and decreasing catabolism (Fig. 1). As the most important signaling pathway in cellular immunometabolism, understanding PI3K signaling-led metabolic reprogramming provides insight into cancer therapeutic potential of pathway inhibitors [40].
PI3K
PI3K is an intracellular phosphatidylinositol kinase in a dimeric structure with serine/threonine (Ser/Thr) kinase active [41]. According to the structure and substrate specifics, PI3K is classified into classes I, II, and III [42]. The type IA PI3K catalytic subunit includes three proteins, p110α, p110β, and p110δ, and the type IB PI3K catalytic subunit, p110γ [43, 44]. The signaling pathway consisting of PI3K and its downstream signaling molecules are essential for cell viability, cycling, metabolism, and other physiological functions in mammals [45]. This also allows signaling pathways mediated by PI3K relevant to multiple disease areas such as tumor therapy, cellular metabolism, inflammation genesis, and immunity [46]. PI3K is activated by signals from receptor tyrosine kinases or G protein-coupled receptors [47]. Upon receipt of an upstream signal, PI3K phosphorylates phosphatidylinositol-4,5-bisphosphate (PIP2), producing large amounts of phosphatidylinositol-3,4,5-trisphosphate (PIP3), thereby recruiting protein kinase-1 (PDK1), phosphorylating the 308 site that activates AKT [48, 49]. In addition, PDK1 can also activate mTORC2, which activates AKT signaling downstream by acting on the tryptophan 473 sites.
As PI3K is widely associated with many intra-organic processes, they have a close influence on both cellular metabolic processes and the immune system, and the activation of PI3K can be involved in multiple biological processes of immune cell development, activation or migration [50]. On one hand, PI3K activity affects the maturation and function of T and B cells [51]. Klaus Okkenhaug and colleagues found loss of PI3Kδ function in B and T cells in a mouse model of p110δ mutation, which leads to impaired T and B-cell antigen receptor signaling and diminished immune responses in vivo, and these mutations in p110δ contribute to the combined immunodeficiency syndrome [52]. PI3K activity also affects neutrophils, macrophages, NK cells, and dendritic cells (DCs) [53, 54]. First, the generation of reactive oxygen species (ROS) by neutrophils to kill microorganisms is dependent on p110γ and p110βPI3K [55]. Second, NK cell maturation and function are critically related to p110δ and p110γ, including cytokine secretion and cytotoxicity. Third, PI3K creates a TME favorable for tumor growth mainly by promoting tumor-associated macrophages (TAMs), regulatory T cells (Tregs), and myeloid-derived suppressor cells (MDSCs), where PI3Kδ dominates the immunosuppressive function of Tregs and MDSCs [56]. In addition, PI3K signaling is specifically vital in tumor angiogenesis. Given the multiple effects of PI3K on TME, PI3K inhibitors promote the proliferation of anti-tumor cells and the infiltration of immune cells to a certain extent, facilitating a positive immunomodulatory efficacy.
PI3K is invariably widely overactivated in cancer and immune dysregulation, manifested by a significant correlation between enhanced tumor microvessel density and increased invasiveness of tumor cells. This has led researchers to look at developing therapeutic PI3K inhibitors, and despite drug resistance and tolerance issues, some PI3K inhibitors have been approved for marketing. Initial studies always focused on pan-PI3K inhibitors. However, as research progressed, excessive toxicities were an important factor limiting the development of such inhibitors, with Bayer’s targeting PI3Kα and PI3Kδ Copanlisib coming last and being approved for recurrent follicular lymphoma in 2017 [57, 58]. And of course, Duvelisib is a PI3Kδ and PI3Kγ inhibitor for lymphoma, which was launched in 2018 [59]. PI3K inhibitors with subtype specificity stand out in this research dilemma, mainly alpelisib, a PI3Kα inhibitor launched in 2019 [60,61,62]. Idelalisib, a PI3Kδ inhibitor for chronic lymphatic leukemia, launched in 2014 [63, 64], and many drugs in clinical studies, PI3K-mTOR, and other multi-target inhibitors are listed below. Specifically, PI3K inhibitors exert efficacy in the following ways. For example, first, since a lack of PI3Kδ and PI3Kγ is associated with impaired immune responses and B-cell development, inhibition of signaling from B-cell receptors can be useful in B-cell lymphoma [65]. Second, some studies have used PI3Kγ inhibitors IPI-549 and silymarin to target tumor-associated fibroblasts to exert anti-cancer activities, as evidenced by significant reductions in Treg and MDSCs, as well as suppression of angiogenesis and the formation of collagen in tumor tissue [66]. Third, given that PI3Kδ dominates the immunosuppressive function of Tregs and MDSCs as described above, PI3Kδ inhibitors may contribute to a positive immune environment and promote cytotoxic T-cell responses [56, 67]. Overall, the reliance of regulatory immune cells upon the PI3K pathway could be treated with PI3K inhibitors to release immunosuppression and restore CD8+ T-cell activity (Table 1).
AKT
AKT, also called PKB, is a Ser/Thr kinase consisting of three allomorphic forms [68]. When the Thr308 and Ser473 sites of AKT are fully activated by phosphorylation of PIP3 and mTORC2, thereby affects a series of downstream substrates, resulting in increased anabolism and decreased catabolism [69]. Specifically, first, AKT inhibits the negative regulatory effect of AKT substrate of 160 kDa (AS160) on glucose transporter 4 (GLUT4), allowing cells to translocate GLUT4-containing vesicles and permit glucose to enter the cell for glycolysis [70]. Second, AKT inhibits GSK3, which relieves the inhibition of glycogen synthase and promotes glycogen synthesis, allowing cells to take up glucose more easily. Third, AKT phosphorylates and activates ACLY to promote fatty acid synthesis. Fourth, AKT inhibits tuberous sclerosis complex 1/2 (TSC1/2) through phosphorylation and unbinds the Ras homolog enriched in the brain (RHeb), allowing RHeb to activate mTORC1 [71].
AKT affects the immune system in two major ways. First, the Akt pathway regulates the activation phenotype of macrophages and modulates macrophage responses through inflammatory and metabolic signaling [72]. Macrophages are classified into M1-type and M2-type [73]. M1-type macrophages are involved in positive immune responses and perform immune surveillance functions. In contrast, the weak antigen-presenting capacity and the secreted suppressive cell factors of M2-type macrophages mediate immune suppression, in which AKT may function. Second, AKT acts as a protector in regulating the evolution of memory CD8+ T-cell responses. As found by Anne Rogel and others, AKT has a crucial role in the immune surveillance of memory CD8+ T cells, as demonstrated that the deficiency of AKT affects the survival of effector CD8+ T cells on conversion to memory CD8+ T cells, leading to a reduction in the number of memory CD8+ T cells and weakening secondary immunity. Also, weakened AKT leads to a deficiency of certain tumor-fighting effector cell types in memory CD8+ T cells, which leads to a reduced ability and lessened effectiveness against tumors [74].
AKT directly influences numerous tumorigenic processes. Given the great importance of AKT, it is a promising therapeutic target, and several AKT inhibitors are under clinical research [75]. Currently, there are allosteric AKT inhibitors in clinical trials, such as MK-2206, BAY1125976, and miransertib, as well as ATP-competitive inhibitors, such as capivasertib and ipatasertib [76, 77]. ATP-competitive inhibitors directly target the kinase structural domain to inhibit its activity. Activating mutations and abnormal expression of the AKT pathway are related to the genesis of many types of cancers, such as breast and lung cancers [78, 79]. A natural product modifier from Brassica vegetables, 3-chloroacetylindole, established as a valid non-competitive AKT1 and AKT2 inhibitor, proved to suppress colorectal cancer cell growth and trigger apoptosis both in vivo and in vitro [80] (Table 2).
mTOR
Mammalian targets proteins of rapamycin, mTOR, including mTORC1 and mTORC2, which is the major regulators of cellular metabolism. Multiple studies have shown that mTORC1 activation is associated with metabolic reprogramming [81]. First, mTORC1 phosphorylation activates p70 ribosomal protein S6 kinase (P70S6K), the most important signaling hub downstream of it signaling pathway, to promote intracellular pyrimidine synthesis, peptide translation synthesis, peptide chain extension, and other pathways leading to increased protein synthesis. Second, mTORC1 could also synergistically increase protein synthesis by inhibiting 4E-binding protein 1 (4EBP1) through phosphorylation so that eukaryotic translation initiation factor 4E (eIF4E) could activate the S6 ribosomal subunit and activate ribosomes [82, 83]. Finally, mTORC1 inhibits cellular autophagy by inhibiting unc-51-like kinase-1 (ULK1), an important initiator that controls autophagosome production and maturation, through phosphorylation.
mTOR has a variety of immunological functions, primarily modulating the differentiation and function of immune cells and also having crucial functions in memory cell development. On one hand, mTOR regulates the differentiation, survival, and metabolic reprogramming of T-cell subsets [84]. On the other hand, mTOR determines the proliferation and maturation of Treg, Th17 cells, and NK cells as well as influences effector function and cytotoxicity [85]. Additionally, mTOR serves a crucial role in regulating cell death, mainly in autophagy, ferroptosis, and scorching death. Given the dual role of autophagy in suppressing cancer at an early stage while maintaining tumor metabolism, growth, and survival promoting tumorigenesis at a later stage. Therefore, inhibiting autophagy with mTOR inhibitors at specific times may increase the metabolic stress on cancer cells to facilitate cell death. The oncogenic activation of the PI3K/AKT/mTORC1 pathway can also inhibit ferroptosis in cancer cells through downstream SREBP1/scd1-mediated adipogenesis, so the combination of mTOR inhibitors and other ferroptosis inducers for cancer treatment may be an excellent therapeutic target [86]. Experiments by Wang, Y. demonstrated a novel mechanism by which mTORC2 signaling promoted the long-lasting maintenance of memory CD4+ T cells by inhibiting the onset of ferroptosis [87]. This study further demonstrated the major form of memory CD4+ T-cell ferroptosis in the presence of mTORC2 deficiency through knockdown and overexpression experiments of GPX4, a key enzyme of the ferroptosis pathway. A new study conducted by Evavold and others found that mTORC1 promoted gasdermin D-mediated inflammatory cell death by controlling ROS production in mitochondria [88, 89].
Dysregulated mTOR activity can be found in a variety of cancers, including prostate, breast, lung, melanoma, bladder, brain, and kidney cancers, leading to mTOR as a critical therapeutic target. The mTOR inhibitor blocks signaling producing positive effects of anti-inflammatory, anti-tumor cell proliferation, and inducing apoptosis. Currently, mTOR inhibitors proceed to the third generation. Sirolimus (rapamycin), everolimus, tesilimus, lidaformycin, and zotamox are the first-generation inhibitors of mTOR, and they are called rapamycin and its derivatives. First-generation mTOR inhibitors, mainly inhibit the complex mTORC1, which may lead to compromised negative feedback on the PI3K signaling pathway, which in turn enhances the phosphorylation activity of AKT, making patients susceptible to drug resistance with the drug. Second-generation inhibitors competitively inhibit mTOR kinases, including mTORC1 and mTORC2. This class of inhibitors blocks the feedback regulation of AKT activation formation caused by mTORC1 inhibitors, so this class of drugs has a stronger inhibitory effect than mTORC1 inhibitors. Rapalink-1, a third-generation mTOR inhibitor, is a linkage of first-and second-generation mTOR inhibitors that can target both targets on the mTOR enzyme. The functions of mTOR signaling in immune cell regulation are diverse, on the one hand promoting T-cell accumulation and clearance in tumors, yet on the other hand, mediating tumor malignancy development and immune evasion. Tumor cells utilize this pathway for vicious progression potentially providing opportunities for the development of T-cell-based immunotherapies. Similarly, mTOR inhibitors can greatly promote or inhibit T-cell chemokine-mediated chemotaxis in TME or inflammation. Although the anti-tumor effect of mTOR signaling and chemokine/receptor axis in mediating immune cells or tumor cells is two-sided, whose effect it has remains an open question (Tables 3 and 4).
GSK3
Glycogen synthase kinase-3 (GSK-3) is also a downstream target of AKT [90, 91]. AKT phosphorylates and inhibits GSK-3, then targets proteasomal degradation to promote glycogen synthesis [92, 93]. Studies now show that GSK-3 is anomalously regimented in various cancers and has oncogenic effects. Thus GSK-3 is emerging as a possible curative candidate for cancer [94, 95]. Although some GSK-3 inhibitors have shown poor efficacy in studies, there is also evidence that they can inhibit the growth of certain cancers [96]. For example, Frank Cichocki and others demonstrated that in the existence of GSK3 inhibitor CHIR99021, the production of tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) by NK cells was significantly increased, which enhanced NK cytotoxicity, fueled immunotherapy of cancer [97]. Furthermore, research has also shown that downregulation of GSK-3 expression using siRNA or inhibition of GSK-3 expression with small molecule inhibitors both downregulate PD-1 levels to augment the killing capacity in CD8+ T cells [98].
ACLY
ATP-citrate lyase (ACLY) is a key enzyme that catalyzes the synthesis of fatty acids and is the major enzyme responsible for the production of acetyl coenzyme A at the cell membrane in many tissues [99, 100]. As is known, malignantly accreting tumor cells exhibit a great demand for lipids and a significant upregulation of fatty acid biosynthetic pathways in various tumor cells [101, 102]. Existing research indicates that ACLY is expressed highly in a wide range of cancers, including colorectal, liver, gastric, and prostate cancers, making ACLY a potentially effective therapeutic target for cancer by affecting lipid metabolism [102,103,104,105,106]. Georgia Hatzivassiliou et al. demonstrated that the knocking down of ACLY, as a key enzyme integrating glucose and lipid metabolism, limited the growth and survival of aerobic glycolytic tumor cells in vitro and reduced tumor development in vivo [107]. Given the key function in lipid metabolism, ACLY inhibitors were formerly developed for metabolic diseases. However, in recent years, ACLY inhibitors have attracted attention as promising anti-cancer drugs as more and more evidence suggests that cancer is a metabolic disease as well as a genetic one [108, 109]. For example, the ACLY inhibitor bempedoic acid (ETC-1002) was already authorized by the U.S. Food and Drug Administration (FDA) in 2020 as a non-statin LDL-C lowering drug for atherosclerotic cardiovascular disease, as well as being used in cancer treatment therapeutics [110, 111].
Taken together, enhanced PI3K/Akt/mTOR signaling induces metabolic alterations within the TME that complexly affect immune cell proliferation and growth. A multitude of different inhibitors of this pathway are in various stages of clinical trials, but only a few of them have been approved by the FDA for use in cancer therapy. In the long run, improving the targeting and sensitivity of inhibitors will further contribute to the advancement of human cancer therapy.
LKB1-AMPK signaling pathway
Another key signaling pathway linking cellular metabolism to carcinogenesis is LKB1-AMPK. LKB1 is a tumor suppressor Ser/Thr kinase that is broadly associated with cellular metabolism and proliferation, as well as modulating various cellular physiopathological processes [112]. AMPK consists of an α catalytic subunit and a β and γ regulatory subunit, which is an important hub for sensing and regulating the homeostasis of cellular energy metabolism [113,114,115]. When AMP binds to the γ subunit, the activation complex can be modified to make it a more susceptible substrate for phosphorylation at the threonine 172 site [116]. In addition to this, the increase in intracellular Ca2+ by calcium/calmodulin-dependent protein kinase kinase 2 (CAMKK2) can also activate AMPK by direct phosphorylation at the threonine 172 site [117,118,119]. When cells are stressed under various physiological conditions resulting in insufficient energy metabolism leading to a decrease in ATP levels, eventually AMPK will also be activated [120, 121]. LKB1-AMPK signaling exerts a core function in mediating cell metabolism, survival, and proliferation under energy stress. This is mainly manifested by the modulation of protein, lipid, and glucose metabolism in mammals, along with autophagy and mitochondrial homeostasis, which encompasses almost most of the physiological and metabolic activities of the living organism. As a key physiological energy sensor, AMPK has a series of downstream targets to exert a wide range of regulatory effects [122]. These include promoting catabolism to reduce ATP consumption and reducing anabolism to increase ATP synthesis, thereby maintaining intracellular energy homeostasis [123]. In conclusion, AMPK and mTOR interact to form a complex master metabolic network to control anabolism and catabolism and exert essential functions in the organism (Fig. 2).
AMPK can be activated directly by LKB1 or in response to increased intracellular Ca2+ and lower ATP levels, acting on a range of downstream signaling molecules to inhibit fatty acid synthesis, protein synthesis, gluconeogenesis, promote glucose uptake, promote autophagy, and maintain mitochondrial function.
As mentioned above, AMPK activation results in the downregulation of mTORC1, which conversely activates the expression of autophagy-related proteins [124]. AMPK activation also phosphorylates ULK1 and promotes its activity, activating the autophagic process [125]. In addition to linking immune signaling and cellular metabolism, LKB1 may also regulate mitochondria-related functions [126]. It has also been demonstrated that AMPK negatively regulates ferroptosis by suppressing fatty acid synthesis, as in the study by Ming-Hui Gao and colleagues, who showed that the LKB1-AMPK pathway prevented ferroptosis by inhibiting Acetyl-CoA carboxylase 1 (ACC1), the rate-limiting enzyme of fatty acid biosynthesis [127]. A collaborative study by Boyi Gan et al.‘s team also showed that AMPK could inhibit ACC, thereby reducing the formation of polyunsaturated fatty acids and ultimately suppressing ferroptosis [128]. It is thus clear that activation of AMPK is becoming a recognized therapeutic target for diseases related to metabolic disorders. On the other hand, Lei Bi and others demonstrated that suppression of the LKB1-AMPK pathway enhanced glycolysis in hepatocellular carcinoma cells, which conversely enhanced the stemness in tumor cells, thus allowing them to develop in an uncontrollable direction [129].
LKB1 has a distinct function in the differentiation and function of T cells, serving as a crucial checkpoint that collaborates with AMPK to centrally regulate lymphocyte metabolism and function [130, 131]. LKB1 acts as a critical cytokine for T-cell development and therefore contributes to the growth and survival of thymocytes. Research by Nancie J. MacIver and others confirmed that LKB1 regulated glucose and lipid metabolism in T lymphocytes, while T cells lacking LKB1 exhibited poor metabolic adaptation [132]. In conclusion, disruption of the LKB1-AMPK axis damages T-cell metabolism and over-activates mTORC1 signaling to mediate the expansion of pro-inflammatory T cells. LKB1 also coordinates metabolic resting and anti-tumor immunity of DCs. The study by Yang et al. showed that the LKB1 axis established metabolic rest in DCs to limit Treg over-expansion and Th17 cell compartmentalization, which maintains immune equilibrium or promotes an anti-tumor immune response [133]. Besides, LKB1 maintains the viability and function of Treg cells by mediating the Treg metabolism [134].
Due to the well-established phenotypic effects of AMPK activation on metabolism, AMPK is already recognized to be a promising target for the treatment of metabolic syndrome and cancer [135]. According to the site of action, AMPK activators are divided into direct activators and indirect activators. In general, direct activators directly interact with specific subunits of AMPK to activate AMPK metamorphically, such as the most widely used 5-aminoimidazole-4-carboxamide riboside (AICAR) and Thienopyridone (A-769662). Indirect AMPK activators refer to several modulators that can indirectly activate AMPK by interfering with ATP production or calcium accumulation, mostly of natural plant origin, such as metformin, curcumin, and resveratrol. Given the complex relationship between AMPK and cancer, AMPK activators currently in preclinical and clinical research focus on the treatment of obesity and diabetes, nonalcoholic fatty liver disease, and cardiovascular disease (Table 5).
Several immunometabolic checkpoints
Findings of immune checkpoints provide new targets in cancer therapy and have been demonstrated so in melanoma and non-small cell lung cancer [136]. However, it is still only a fraction of the patients produced significant efficacy. As researchers dug deeper into the mechanism of tumor metabolism, resistance to immune checkpoint therapy may stem from tumor cell-induced dysregulation of immune cell metabolism, which leads to immunosuppression [137]. Therefore, it may be advantageous to use metabolic pathways to kill tumor cells or reverse the metabolic vulnerability of immune cells to target cancer. Recently reported immunometabolic checkpoints with significant potential may provide new insights into anti-tumor therapies.
IDO
IDO, known as indoleamine 2,3-dioxygenase, is responsible for the degradation of tryptophan, which can be metabolized to N-formyl-kynurenine [138]. High expression of IDO is positively associated with poor patient prognosis in various tumor types [139]. IDO promotes “metabolic, immune regulation” through catalytic oxidative catabolism of the essential amino acid tryptophan (Trp) along the kynurenine (Kyn) pathway [140, 141]. Metabolites of the Kyn pathway can exert immunosuppressive effects by acting as natural immunoreactive ligands for the aryl hydrocarbon receptor (AHR), activating Treg, and MDSCs and inhibiting immune cell functions such as effector T cells [142, 143]. Immune cells are highly dependent on Trp, and Trp depletion caused by IDO overexpression leads to an inadequate immune response [144]. Moreover, a study by Xin Zhang et al. in colitis-associated colorectal cancer showed that Treg-induced immune tolerance could be suppressed by inhibiting IDO expression and activation in tumor cells [145]. While long-standing research on IDO has focused on its ability to deplete Trp for immunosuppressive effects, Peter J Murray’s team discovered a new mechanism by which IDO promoted tumor development by transporting the IDO metabolite Kyn into cells via SLC7A11 and inhibiting ferroptosis in the tumor [146]. Up to now, IDO inhibitors (e.g., navoximod, epacadostat, linrodostat, indoximod) are being used as immunomodulators alone or in combination with other anti-tumor therapies [147, 148]. In conclusion, deeper research on existing small molecule compounds, discovering more effective IDO1 inhibitors, and improving the efficacy of combination therapy are the main research areas for IDO inhibitors.
IL4I1
AHR is a ligand-activated transcription factor conferring flexibility to cells in sensing changes in conditions such as environment, diet, metabolites, and microbial composition [149]. AHR was initially thought to be a mediator of dioxin exerting toxicity, and as research progressed, it also proved to exert a major action in cancer and immunity [150]. As mentioned above, IDO functioned in activating AHR by depleting Trp and accumulating Kyn through the Kyn pathway. However, the difficult progress made with the combination of IDO inhibitors and immune checkpoint blockade therapies suggests that there may be other pathways of AHR activation that led to mechanisms of tolerance to IDO inhibitors in tumor cells. A study by Christiane A. Opitz and colleagues identified, through screening and analyzing a broad range of tumor cases, the highest correlation between IL4I1 and AHR activity. The results suggest that IL4I1 is a tumor-produced metabolic enzyme that mainly catabolizes tryptophan to activate AHR, enhancing tumor aggressiveness and inhibiting anti-tumor immunity [151, 152]. The available findings suggest that IL4I1 changes the anti-tumor CD8+ T-cell response, promotes cancer growth, affects patient survival, and may inhibit immune checkpoint inhibitor therapeutic efficacy. So, IL4I1 may be a well-established immunometabolic checkpoint [153,154,155].
ACAT
ACAT is an acetyl coenzyme A cholesterol acetyltransferase that converts cholesterol to cholesteryl esters through the acetylation of cholesterol [156, 157]. In mammals, two genes encoding ACAT1 and ACAT2 were identified, and ACATs act as essential players in cellular cholesterol homeostasis [158]. It has been shown that ACAT2 was induced in some HCC tissues to establish specific cholesterol metabolic pathways for tumor cells, which in turn inhibits anti-tumor immunity [159]. Mala K. Maini et al. also found that using ACAT inhibitors to regulate cholesterol metabolism may have the unique function of directly targeting viruses and tumors, while also enhancing the clearing of viruses by T cells [160]. In addition, studies by Wei Yang and others demonstrated inhibition of ACAT1 activity increased the cholesterol levels in CD8+ T-cell membranes, enhanced the signaling of these killer T cells, formed more effective immune synapses, and resulted in greater sensitivity to antigens and in turn improved immune efficacy [161]. ACAT inhibitors are cholesterol-modulating drugs such as Avasimibe that are well tolerated in clinical trials as cholesterol-lowering agents, and available studies indicate that the combined use of Avasimibe and PD-1 antibodies may improve the efficacy of tumor immunotherapy even more. ACAT appears to be an attractive metabolic regulatory target, and blocking the cholesterol metabolic pathway mediated by ACAT may have a potential therapeutic effect in cancer patients.
SIRT2
Sirtuin 2 (SIRT2) is a member of the Sirtuin family of proteins. SIRT2 is a NAD + -dependent deacetylase with proven critical functions in neurodegenerative diseases. Still, some reports of a dual paradoxical role of cancer promotion and inhibition have hindered its in-depth study [162, 163]. SIRT2 is involved in the control of cell metabolism, cell cycle, and TME [164]. Now, SIRT2 has been found to act as a key immunometabolic checkpoint for reprogramming T metabolism. Imene Hamaidi et al. found that expression of increased glycolysis and oxidative phosphorylation of T cells in SIRT2-deficient mice resulted in increased T-cell proliferation and killing capacity, which subsequently exhibited superior anti-tumor activity. The results presented that SIRT2 suppression promotes metabolic reprogramming of T cells optimally involved in aerobic glycolysis and mitochondrial respiration, maintaining T-cell effector functions in metabolically stressed TME [165]. Although the controversy over whether SIRT2 is cancer-promoting or cancer-suppressing still exists, according to some reports, SIRT2 inhibitors have shown real promise in treating cancer [166,167,168].
MTHFD2
Methylenetetrahydrofolate dehydrogenase (MTHFD2) is the essential enzyme in cellular one-carbon unit metabolism, catalyzing the generation of methylenetetrahydrofolate to formyltetrahydrofolate by using NADP+ as the hydrogen acceptor and generating NADPH [169, 170]. Folate metabolism is a key metabolic process in organisms, providing folate intermediates to promote single-carbon metabolism, in which alterations in folate metabolism or upregulated expression of single-carbon metabolizing enzymes are thought to be involved in a higher risk of cancer [171, 172]. Overexpression of MTHFD2 in various types of cancer cells enhancing PD-L1 expression and increasing immune escape of tumor cells was studied [173, 174]. In addition, MTHFD2 expression is closely associated with mTORC1 signaling, which controls protein, lipid, and nucleotide synthesis in normal and cancer cells [175]. Recently, MTHFD2 was reported to be a metabolic checkpoint of cells that inhibits anti-inflammatory Treg cells and is a potential therapeutic target within 1 C metabolism. Mechanistically, inhibition of MTHFD2 leads to exhaustion of the purine pool, accumulation of purine biosynthetic intermediates and reduction of the trophic sensor mTORC1 signaling [176]. Therefore, in conclusion, MTHFD2 is to some extent oncogenic, which could be considered a therapeutic target and prognostic indicator for cancer, and future research directions should profoundly elucidate the mechanism of its promotion of cancer progression and accelerate the progress of functional inhibitors [177,178,179].
Concluding remarks
With various immune checkpoint inhibitors (ICIs) and chimeric antigen receptor T-cell therapies led by PD-1/PD-L1 being developed, immunotherapy can be considered to have transformed the treatment of multiple cancers to some extent [180]. Still, the response rates of patients vary widely to the available immune-targeted therapies, including frequent resistance to immune checkpoint therapies [181]. A growing number of immunometabolic studies show a promising trend to increase anti-cancer effects through metabolic targets, and immunometabolism offers a broad opportunity to improve cancer therapy by modulating TME to identify new targets [182]. As the Warburg effect is well understood, reprogramming of the metabolism of the cancer cells drives the metabolic dysregulation of TME, causing partial failure of T-cell-based cancer immunotherapy. Increasing evidence suggests that the metabolic adaptations of T cells determine their function, as mentioned above, by reprogramming T-cell metabolism through checkpoints like IDO, IL4I1, and SIRT2, resulting in improved anti-cancer immune efficacy. In addition, Guo et al. found that IL-10/Fc can produce effective T-cell metabolic reprogramming through upregulation of oxidative phosphorylation, which could rejuvenate depleted T cells and augment the cancer immunotherapy response [183]. Therefore, identifying mechanisms of metabolic vulnerability of immune cells and conferring exogenous flexibility to restore the killing effect on tumor cells also seems to be a more feasible approach.
We have described the key signaling pathways of immunometabolism and some recently reported immunometabolic checkpoints, including how they affect the function and fate of immune cells through metabolic pathways, which in turn regulate the tumor immune process. On the one hand, perhaps the focus of future immunometabolic research will be to develop highly effective targeted drugs that combine specificity and safety or to improve cancer immunotherapy in combination with ICIs to improve drug resistance. On the other hand, existing immunometabolism research lacks a profound revelation of the functional and molecular regulatory mechanisms of abnormal immune cell metabolism in TME, making the clinical treatment of immunometabolism lack potential new strategies and targets.
To date, there has been growing research on the integration of the metabolic and immune domains, but there is a lack of clinical trials evaluating the metabolic interactions between immune and cancer cells assessed in human tumors. Tumor cell metabolism with heterogeneity impairs anti-tumor immunity to some extent, so clinical studies should confer metabolic advantages to immune cell populations through various pathways to better improve cancer treatment and patient prognosis. We seek to enhance the understanding of the multifaceted functions of complex immunometabolic signaling pathways in the TME and to gain a deeper understanding of immunometabolism, which we hope will benefit cancer immunotherapy.
Data availability
All data generated or analyzed during this study are included in this article.
References
Malik D, Mahendiratta S, Kaur H, Medhi B. Futuristic approach to cancer treatment. Gene. 2021;805:145906.
Finn OJ. Immuno-oncology: understanding the function and dysfunction of the immune system in cancer. Ann Oncol. 2012;23:viii6–9.
Saravia J, Raynor JL, Chapman NM, Lim SA, Chi H. Signaling networks in immunometabolism. Cell Res. 2020;30:328–42.
Singer K, Cheng WC, Kreutz M, Ho PC, Siska PJ. Immunometabolism in cancer at a glance. Dis Model Mech. 2018;11:dmm034272.
Li X, Wenes M, Romero P, Huang SC, Fendt SM, Ho PC. Navigating metabolic pathways to enhance antitumour immunity and immunotherapy. Nat Rev Clin Oncol. 2019;16:425–41.
Kim J, DeBerardinis RJ. Mechanisms and implications of metabolic heterogeneity in cancer. Cell Metab. 2019;30:434–46.
Chang CH, Qiu J, O’Sullivan D, Buck MD, Noguchi T, Curtis JD, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162:1229–41.
Anderson NM, Simon MC. The tumor microenvironment. Curr Biol. 2020;30:R921–R925.
Soysal SD, Tzankov A, Muenst SE. Role of the tumor microenvironment in breast cancer. Pathobiology. 2015;82:142–52.
Guo J, Wang M-F, Zhu Y, Watari F, Xu Y-H, Chen X. Exploitation of platelets for antitumor drug delivery and modulation of the tumor immune microenvironment. Acta Materia Medica. 2023;2:172–190.
Fendt SM, Frezza C, Erez A. Targeting metabolic plasticity and flexibility dynamics for cancer therapy. Cancer Discov. 2020;10:1797–807.
Jung JG, Le A. Metabolism of immune cells in the tumor microenvironment. Adv Exp Med Biol. 2021;1311:173–85.
Poznanski SM, Singh K, Ritchie TM, Aguiar JA, Fan IY, Portillo AL, et al. Metabolic flexibility determines human NK cell functional fate in the tumor microenvironment. Cell Metab. 2021;33:1205–20.e1205.
García-Caballero M, Sokol L, Cuypers A, Carmeliet P. Metabolic reprogramming in tumor endothelial cells. Int J Mol Sci. 2022;23:11052.
Warburg O. On the origin of cancer cells. Science. 1956;123:309–14.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33.
Kalyanaraman B. Teaching the basics of cancer metabolism: developing antitumor strategies by exploiting the differences between normal and cancer cell metabolism. Redox Biol. 2017;12:833–42.
Icard P, Shulman S, Farhat D, Steyaert JM, Alifano M, Lincet H. How the Warburg effect supports aggressiveness and drug resistance of cancer cells? Drug Resist Updat. 2018;38:1–11.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Reina-Campos M, Moscat J, Diaz-Meco M. Metabolism shapes the tumor microenvironment. Curr Opin Cell Biol. 2017;48:47–53.
Maddocks OD, Berkers CR, Mason SM, Zheng L, Blyth K, Gottlieb E. et al. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature. 2013;493:542–6.
Andrejeva G, Rathmell JC. Similarities and distinctions of cancer and immune metabolism in inflammation and tumors. Cell Metab. 2017;26:49–70.
Buck MD, O’Sullivan D, Pearce EL. T cell metabolism drives immunity. J Exp Med. 2015;212:1345–60.
Bader JE, Voss K, Rathmell JC. Targeting metabolism to improve the tumor microenvironment for cancer immunotherapy. Mol Cell. 2020;78:1019–33.
Geltink RIK, Kyle RL, Pearce EL. Unraveling the complex interplay between T cell metabolism and function. Annu Rev Immunol. 2018;36:461–88.
Madden MZ, Rathmell JC. The complex integration of T-cell metabolism and immunotherapy. Cancer Discov. 2021;11:1636–43.
Chang CH, Curtis JD, Maggi LB, Faubert B, Villarino AV, O’Sullivan D. et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153:1239–51.
Xia L, Oyang L, Lin J, Tan S, Han Y, Wu N. et al. The cancer metabolic reprogramming and immune response. Mol Cancer. 2021;20:28.
Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4 + T cell subsets. J Immunol. 2011;186:3299–303.
Kishton RJ, Sukumar M, Restifo NP. Metabolic regulation of T cell longevity and function in tumor immunotherapy. Cell Metab. 2017;26:94–109.
Gerriets VA, Kishton RJ, Nichols AG, Macintyre AN, Inoue M, Ilkayeva O, et al. Metabolic programming and PDHK1 control CD4 + T cell subsets and inflammation. J Clin Invest. 2015;125:194–207.
Scharping NE, Menk AV, Moreci RS, Whetstone RD, Dadey RE, Watkins SC. et al. The tumor microenvironment represses T cell mitochondrial biogenesis to drive intratumoral T cell metabolic insufficiency and dysfunction. Immunity. 2016;45:701–3.
Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–70.
MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol. 2013;31:259–83.
Miricescu D, Totan A, Stanescu-Spinu II, Badoiu SC, Stefani C, Greabu M. PI3K/AKT/mTOR Signaling Pathway in Breast Cancer: From Molecular Landscape to Clinical Aspects. Int J Mol Sci. 2020;22:173.
Zhu K, Wu Y, He P, Fan Y, Zhong X, Zheng H, et al. PI3K/AKT/mTOR-targeted therapy for breast cancer. Cells. 2022;11:2508.
Chen L, Zhang W, Chen D, Yang Q, Sun S, Dai Z, et al. RBM4 dictates ESCC cell fate switch from cellular senescence to glutamine-addiction survival through inhibiting LKB1-AMPK-axis. Signal Transduct Target Ther. 2023;8:159.
Chou WC, Rampanelli E, Li X, Ting JP. Impact of intracellular innate immune receptors on immunometabolism. Cell Mol Immunol. 2022;19:337–51.
Aoki M, Fujishita T. Oncogenic roles of the PI3K/AKT/mTOR axis. Curr Top Microbiol Immunol. 2017;407:153–89.
Park JH, Pyun WY, Park HW. Cancer metabolism: phenotype, signaling and therapeutic targets. Cells. 2020;9:2308.
Morgos DT, Stefani C, Miricescu D, Greabu M, Stanciu S, Nica S, et al. Targeting PI3K/AKT/mTOR and MAPK signaling pathways in gastric cancer. Int J Mol Sci. 2024;25:1848.
Pozo FM, Hunter T, Zhang Y. The ‘New (Nu)-clear’ evidence for the tumor-driving role of PI3K. Acta Mater Med. 2022;1:193–6.
De Santis MC, Gulluni F, Campa CC, Martini M, Hirsch E. Targeting PI3K signaling in cancer: challenges and advances. Biochim Biophys Acta Rev Cancer. 2019;1871:361–6.
Shorning BY, Dass MS, Smalley MJ, Pearson HB. The PI3K-AKT-mTOR pathway and prostate cancer: at the crossroads of AR, MAPK, and WNT signaling. Int J Mol Sci. 2020;21:4507.
Glaviano A, Foo ASC, Lam HY, Yap KCH, Jacot W, Jones RH, et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol Cancer. 2023;22:138.
Tian LY, Smit DJ, Jucker M. The Role of PI3K/AKT/mTOR signaling in hepatocellular carcinoma metabolism. Int J Mol Sci. 2023;24:2652.
Lien EC, Dibble CC, Toker A. PI3K signaling in cancer: beyond AKT. Curr Opin Cell Biol. 2017;45:62–71.
Guerrero-Zotano A, Mayer IA, Arteaga CL. PI3K/AKT/mTOR: role in breast cancer progression, drug resistance, and treatment. Cancer Metastasis Rev. 2016;35:515–24.
Goulden BD, Pacheco J, Dull A, Zewe JP, Deiters A, Hammond GRV. A high-avidity biosensor reveals plasma membrane PI(3,4)P(2) is predominantly a class I PI3K signaling product. J Cell Biol. 2019;218:1066–79.
Chen R, Sun G, Xu L, Zhang X, Zeng W, Sun X. Didymin attenuates doxorubicin-induced cardiotoxicity by inhibiting oxidative stress. Chin Herb Med. 2022;14:70–78.
Calderon L, Schindler K, Malin SG, Schebesta A, Sun Q, Schwickert T. et al. Pax5 regulates B cell immunity by promoting PI3K signaling via PTEN down-regulation. Sci Immunol. 2021;6:eabg5003.
Lucas CL, Chandra A, Nejentsev S, Condliffe AM, Okkenhaug K. PI3Kdelta and primary immunodeficiencies. Nat Rev Immunol. 2016;16:702–14.
Kulkarni S, Sitaru C, Jakus Z, Anderson KE, Damoulakis G, Davidson K, et al. PI3Kbeta plays a critical role in neutrophil activation by immune complexes. Sci Signal. 2011;4:ra23.
Kaneda MM, Cappello P, Nguyen AV, Ralainirina N, Hardamon CR, Foubert P, et al. Macrophage PI3Kgamma drives pancreatic ductal adenocarcinoma progression. Cancer Discov. 2016;6:870–85.
Hao L, Lei X, Zhou H, Marshall AJ, Liu L. Critical role for PI3Kgamma-dependent neutrophil reactive oxygen species in WKYMVm-induced microvascular hyperpermeability. J Leukoc Biol. 2019;106:1117–27.
Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13:140–56.
Magagnoli M, Carlo-Stella C, Santoro A. Copanlisib for the treatment of adults with relapsed follicular lymphoma. Expert Rev Clin Pharm. 2020;13:813–23.
Munoz J, Follows GA, Nastoupil LJ. Copanlisib for the treatment of malignant lymphoma: clinical experience and future perspectives. Target Oncol. 2021;16:295–308.
Blair HA. Duvelisib: first global approval. Drugs. 2018;78:1847–53.
Andre F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019;380:1929–40.
Andre F, Ciruelos EM, Juric D, Loibl S, Campone M, Mayer IA, et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: final overall survival results from SOLAR-1. Ann Oncol. 2021;32:208–17.
Turner S, Chia S, Kanakamedala H, Hsu WC, Park J, Chandiwana D, et al. Effectiveness of alpelisib + fulvestrant compared with real-world standard treatment among patients with HR + , HER2-, PIK3CA-mutated breast cancer. Oncologist. 2021;26:e1133–42.
Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007.
Shah A, Mangaonkar A. Idelalisib: a novel PI3Kdelta inhibitor for chronic lymphocytic leukemia. Ann Pharmacother. 2015;49:1162–70.
Seiler T, Hutter G, Dreyling M. The emerging role of PI3K inhibitors in the treatment of hematological malignancies: preclinical data and clinical progress to date. Drugs. 2016;76:639–46.
Jiang M, He K, Qiu T, Sun J, Liu Q, Zhang X, et al. Tumor-targeted delivery of silibinin and IPI-549 synergistically inhibit breast cancer by remodeling the microenvironment. Int J Pharm. 2020;581:119239.
LoRusso PM. Inhibition of the PI3K/AKT/mTOR pathway in solid tumors. J Clin Oncol. 2016;34:3803–15.
Shariati M, Meric-Bernstam F. Targeting AKT for cancer therapy. Expert Opin Investig Drugs. 2019;28:977–88.
Song M, Bode AM, Dong Z, Lee MH. AKT as a therapeutic target for cancer. Cancer Res. 2019;79:1019–31.
Revathidevi S, Munirajan AK. Akt in cancer: mediator and more. Semin Cancer Biol. 2019;59:80–91.
Shackelford DB, Shaw RJ. The LKB1–AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563–75.
Vergadi E, Ieronymaki E, Lyroni K, Vaporidi K, Tsatsanis C. Akt signaling pathway in macrophage activation and M1/M2 polarization. J Immunol. 2017;198:1006–14.
Covarrubias AJ, Aksoylar HI, Horng T. Control of macrophage metabolism and activation by mTOR and Akt signaling. Semin Immunol. 2015;27:286–96.
Rogel A, Willoughby JE, Buchan SL, Leonard HJ, Thirdborough SM, Al-Shamkhani A. Akt signaling is critical for memory CD8( + ) T-cell development and tumor immune surveillance. Proc Natl Acad Sci USA. 2017;114:E1178–E1187.
Brown JS, Banerji U. Maximising the potential of AKT inhibitors as anti-cancer treatments. Pharm Ther. 2017;172:101–15.
Narayan RS, Fedrigo CA, Brands E, Dik R, Stalpers LJ, Baumert BG, et al. The allosteric AKT inhibitor MK2206 shows a synergistic interaction with chemotherapy and radiotherapy in glioblastoma spheroid cultures. BMC Cancer. 2017;17:204.
Halder AK, Cordeiro M. AKT inhibitors: the road ahead to computational modeling-guided discovery. Int J Mol Sci. 2021;22:3944.
Shi Y, Liu X, Han EK, Guan R, Shoemaker AR, Oleksijew A, et al. Optimal classes of chemotherapeutic agents sensitized by specific small-molecule inhibitors of AKT in vitro and in vivo. Neoplasia. 2005;7:992–1000.
Nitulescu GM, Margina D, Juzenas P, Peng Q, Olaru OT, Saloustros E, et al. AKT inhibitors in cancer treatment: the long journey from drug discovery to clinical use (review). Int J Oncol. 2016;48:869–85.
Kim DJ, Reddy K, Kim MO, Li Y, Nadas J, Cho YY, et al. (3-Chloroacetyl)-indole, a novel allosteric AKT inhibitor, suppresses colon cancer growth in vitro and in vivo. Cancer Prev Res (Philos.). 2011;4:1842–51.
Mossmann D, Park S, Hall MN. mTOR signalling and cellular metabolism are mutual determinants in cancer. Nat Rev Cancer. 2018;18:744–57.
Kim YC, Guan KL. mTOR: a pharmacologic target for autophagy regulation. J Clin Invest. 2015;125:25–32.
Braun C, Weichhart T. mTOR-dependent immunometabolism as Achilles’ heel of anticancer therapy. Eur J Immunol. 2021;51:3161–75.
Powell JD, Pollizzi KN, Heikamp EB, Horton MR. Regulation of immune responses by mTOR. Annu Rev Immunol. 2012;30:39–68.
Rostamzadeh D, Yousefi M, Haghshenas MR, Ahmadi M, Dolati S, Babaloo Z. mTOR signaling pathway as a master regulator of memory CD8( + ) T-cells, Th17, and NK cells development and their functional properties. J Cell Physiol. 2019;234:12353–68.
Yi J, Zhu J, Wu J, Thompson CB, Jiang X. Oncogenic activation of PI3K-AKT-mTOR signaling suppresses ferroptosis via SREBP-mediated lipogenesis. Proc Natl Acad Sci USA. 2020;117:31189–97.
Wang Y, Tian Q, Hao Y, Yao W, Lu J, Chen C, et al. The kinase complex mTORC2 promotes the longevity of virus-specific memory CD4 T cells by preventing ferroptosis. Nat Immunol. 2022;23:303–17.
Evavold CL, Hafner-Bratkovic I, Devant P, D’Andrea JM, Ngwa EM, Borsic E, et al. Control of gasdermin D oligomerization and pyroptosis by the Ragulator-Rag-mTORC1 pathway. Cell. 2021;184:4495-4511.e19.
Liu Z, Xiao TS. Partners with a killer: metabolic signaling promotes inflammatory cell death. Cell. 2021;184:4374–6.
Hermida MA, Dinesh Kumar J, Leslie NR. GSK3 and its interactions with the PI3K/AKT/mTOR signalling network. Adv Biol Regul. 2017;65:5–15.
Kaidanovich-Beilin O, Woodgett JR. GSK-3: functional insights from cell biology and animal models. Front Mol Neurosci. 2011;4:40.
Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–9.
Duda P, Akula SM, Abrams SL, Steelman LS, Martelli AM, Cocco L, et al. Targeting GSK3 and associated signaling pathways involved in cancer. Cells. 2020;9:1110.
Nagini S, Sophia J, Mishra R. Glycogen synthase kinases: moonlighting proteins with theranostic potential in cancer. Semin Cancer Biol. 2019;56:25–36.
Martelli AM, Evangelisti C, Paganelli F, Chiarini F, McCubrey JA. GSK-3: a multifaceted player in acute leukemias. Leukemia. 2021;35:1829–42.
Roca C, Campillo NE. Glycogen synthase kinase 3 (GSK-3) inhibitors: a patent update (2016-2019). Expert Opin Ther Pat. 2020;30:863–72.
Cichocki F, Valamehr B, Bjordahl R, Zhang B, Rezner B, Rogers P, et al. GSK3 inhibition drives maturation of NK cells and enhances their antitumor activity. Cancer Res. 2017;77:5664–75.
Taylor A, Harker JA, Chanthong K, Stevenson PG, Zuniga EI, Rudd CE. Glycogen synthase kinase 3 inactivation drives T-bet-mediated downregulation of co-receptor PD-1 to enhance CD8(+) cytolytic T cell responses. Immunity. 2016;44:274–86.
Wen J, Min X, Shen M, Hua Q, Han Y, Zhao L, et al. ACLY facilitates colon cancer cell metastasis by CTNNB1. J. Exp Clin Cancer Res. 2019;38:401.
Zaidi N, Swinnen JV, Smans K. ATP-citrate lyase: a key player in cancer metabolism. Cancer Res. 2012;72:3709–14.
Migita T, Narita T, Nomura K, Miyagi E, Inazuka F, Matsuura M, et al. ATP citrate lyase: activation and therapeutic implications in non-small cell lung cancer. Cancer Res. 2008;68:8547–54.
Qian X, Hu J, Zhao J, Chen H. ATP citrate lyase expression is associated with advanced stage and prognosis in gastric adenocarcinoma. Int J Clin Exp Med. 2015;8:7855–60.
Lucenay KS, Doostan I, Karakas C, Bui T, Ding Z, Mills GB, et al. Cyclin E associates with the lipogenic enzyme ATP-citrate lyase to enable malignant growth of breast cancer cells. Cancer Res. 2016;76:2406–18.
Gao Y, Islam MS, Tian J, Lui VW, Xiao D. Inactivation of ATP citrate lyase by Cucurbitacin B: a bioactive compound from cucumber, inhibits prostate cancer growth. Cancer Lett. 2014;349:15–25.
Zhou Y, Bollu LR, Tozzi F, Ye X, Bhattacharya R, Gao G, et al. ATP citrate lyase mediates resistance of colorectal cancer cells to SN38. Mol Cancer Ther. 2013;12:2782–91.
Guo L, Guo YY, Li BY, Peng WQ, Chang XX, Gao X, et al. Enhanced acetylation of ATP-citrate lyase promotes the progression of nonalcoholic fatty liver disease. J Biol Chem. 2019;294:11805–16.
Hatzivassiliou G, Zhao F, Bauer DE, Andreadis C, Shaw AN, Dhanak D, et al. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 2005;8:311–21.
Granchi C. ATP citrate lyase (ACLY) inhibitors: An anti-cancer strategy at the crossroads of glucose and lipid metabolism. Eur J Med Chem. 2018;157:1276–91.
Masoudi-Nejad A, Asgari Y. Metabolic cancer biology: structural-based analysis of cancer as a metabolic disease, new sights and opportunities for disease treatment. Semin Cancer Biol. 2015;30:21–29.
Feng X, Zhang L, Xu S, Shen AZ. ATP-citrate lyase (ACLY) in lipid metabolism and atherosclerosis: an updated review. Prog Lipid Res. 2020;77:101006.
Qiao C, Huang W, Chen J, Feng W, Zhang T, Wang Y, et al. IGF1-mediated HOXA13 overexpression promotes colorectal cancer metastasis through upregulating ACLY and IGF1R. Cell Death Dis. 2021;12:564.
Li TT, Zhu HB. LKB1 and cancer: the dual role of metabolic regulation. Biomed Pharmacother. 2020;132:110872.
Yan Y, Zhou X, Xu H, Melcher K. Structure and physiological regulation of AMPK. Int J Mol Sci. 2018;19:3534.
Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–26.
Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2017;19:121–35.
Yi Y, Chen D, Ao J, Zhang W, Yi J, Ren X, et al. Transcriptional suppression of AMPKalpha1 promotes breast cancer metastasis upon oncogene activation. Proc Natl Acad Sci USA. 2020;117:8013–21.
Ciccarese F, Zulato E, Indraccolo S. LKB1/AMPK pathway and drug response in cancer: a therapeutic perspective. Oxid Med Cell Longev. 2019;2019:8730816.
Li S, Lavagnino Z, Lemacon D, Kong L, Ustione A, Ng X, et al. Ca(2 + )-stimulated AMPK-dependent phosphorylation of Exo1 protects stressed replication forks from aberrant resection. Mol Cell. 2019;74:1123–37.e1126.
Penfold L, Woods A, Muckett P, Nikitin AY, Kent TR, Zhang S, et al. CAMKK2 promotes prostate cancer independently of AMPK via increased lipogenesis. Cancer Res. 2018;78:6747–61.
Endo H, Owada S, Inagaki Y, Shida Y, Tatemichi M. Glucose starvation induces LKB1-AMPK-mediated MMP-9 expression in cancer cells. Sci Rep. 2018;8:10122.
Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Mäkelä TP, et al. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28.
Grahame Hardie D. AMP-activated protein kinase: a key regulator of energy balance with many roles in human disease. J Intern Med. 2014;276:543–59.
Hardie DG, Lin SC. AMP-activated protein kinase—not just an energy sensor. F1000Res. 2017;6:1724.
Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W. et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–61.
Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–41.
Toyama EQ, Herzig S, Courchet J, Lewis TL Jr., Losón OC, Hellberg K, et al. Metabolism. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science. 2016;351:275–81.
Li C, Dong X, Du W, Shi X, Chen K, Zhang W, et al. LKB1-AMPK axis negatively regulates ferroptosis by inhibiting fatty acid synthesis. Signal Transduct Target Ther. 2020;5:187.
Lee H, Zandkarimi F, Zhang Y, Meena JK, Kim J, Zhuang L, et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat Cell Biol. 2020;22:225–34.
Bi L, Ren Y, Feng M, Meng P, Wang Q, Chen W, et al. HDAC11 regulates glycolysis through the LKB1/AMPK signaling pathway to maintain hepatocellular carcinoma stemness. Cancer Res. 2021;81:2015–28.
Blagih J, Krawczyk CM, Jones RG. LKB1 and AMPK: central regulators of lymphocyte metabolism and function. Immunol Rev. 2012;249:59–71.
Andris F, Leo O. AMPK in lymphocyte metabolism and function. Int Rev Immunol. 2015;34:67–81.
MacIver NJ, Blagih J, Saucillo DC, Tonelli L, Griss T, Rathmell JC, et al. The liver kinase B1 is a central regulator of T cell development, activation, and metabolism. J Immunol. 2011;187:4187–98.
Yang K, Blanco DB, Neale G, Vogel P, Avila J, Clish CB. et al. Homeostatic control of metabolic and functional fitness of Treg cells by LKB1 signalling. Nature. 2017;548:602–6.
He N, Fan W, Henriquez B, Yu RT, Atkins AR, Liddle C, et al. Metabolic control of regulatory T cell (Treg) survival and function by Lkb1. Proc Natl Acad Sci USA. 2017;114:12542–7.
Rana S, Blowers EC, Natarajan A. Small molecule adenosine 5’-monophosphate activated protein kinase (AMPK) modulators and human diseases. J Med Chem. 2015;58:2–29.
Giannone G, Ghisoni E, Genta S, Scotto G, Tuninetti V, Turinetto M. et al. Immuno-metabolism and microenvironment in cancer: key players for immunotherapy. Int J Mol Sci. 2020;21:4414
Weng CY, Kao CX, Chang TS, Huang YH. Immuno-metabolism: the role of cancer niche in immune checkpoint inhibitor resistance. Int J Mol Sci. 2021;22:1258
Zhai L, Bell A, Ladomersky E, Lauing KL, Bollu L, Sosman JA, et al. Immunosuppressive IDO in cancer: mechanisms of action, animal models, and targeting strategies. Front Immunol. 2020;11:1185.
Munn DH. Blocking IDO activity to enhance anti-tumor immunity. Front Biosci (Elite Ed.). 2012;4:734–45.
Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34:137–43.
Theate I, van Baren N, Pilotte L, Moulin P, Larrieu P, Renauld JC, et al. Extensive profiling of the expression of the indoleamine 2,3-dioxygenase 1 protein in normal and tumoral human tissues. Cancer Immunol Res. 2015;3:161–72.
Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190–8.
Yentz S, Smith D. Indoleamine 2,3-dioxygenase (IDO) inhibition as a strategy to augment cancer immunotherapy. BioDrugs. 2018;32:311–7.
Brochez L, Chevolet I, Kruse V. The rationale of indoleamine 2,3-dioxygenase inhibition for cancer therapy. Eur J Cancer. 2017;76:167–82.
Zhang X, Liu X, Zhou W, Du Q, Yang M, Ding Y, et al. Blockade of IDO-kynurenine-AhR axis ameliorated colitis-associated colon cancer via inhibiting immune tolerance. Cell Mol Gastroenterol Hepatol. 2021;12:1179–99.
Fiore A, Zeitler L, Russier M, Gross A, Hiller MK, Parker JL, et al. Kynurenine importation by SLC7A11 propagates anti-ferroptotic signaling. Mol Cell. 2022;82:920–32 e927.
Guo Y, Liu Y, Wu W, Ling D, Zhang Q, Zhao P. et al. Indoleamine 2,3-dioxygenase (Ido) inhibitors and their nanomedicines for cancer immunotherapy. Biomaterials. 2021;276:121018.
Le Naour J, Galluzzi L, Zitvogel L, Kroemer G, Vacchelli E. Trial watch: IDO inhibitors in cancer therapy. Oncoimmunology. 2020;9:1777625.
Rothhammer V, Quintana FJ. The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat Rev Immunol. 2019;19:184–97.
Gutiérrez-Vázquez C, Quintana FJ. Regulation of the immune response by the aryl hydrocarbon receptor. Immunity. 2018;48:19–33.
Wang Z, Li T, Mao C, Liu W, Tao Y. IL4I1-driven AHR signature: a new avenue for cancer therapy. Signal Transduct Target Ther. 2021;6:118.
Sadik A, Somarribas Patterson LF, Ozturk S, Mohapatra SR, Panitz V, Secker PF, et al. IL4I1 is a metabolic immune checkpoint that activates the AHR and promotes tumor progression. Cell. 2020;182:1252–70.e1234.
Castellano F, Prevost-Blondel A, Cohen JL, Molinier-Frenkel V. What role for AHR activation in IL4I1-mediated immunosuppression?. Oncoimmunology. 2021;10:1924500.
Zeitler L, Fiore A, Meyer C, Russier M, Zanella G, Suppmann S. et al. Anti-ferroptotic mechanism of IL4i1-mediated amino acid metabolism. eLife. 2021;10:e64806.
Carbonnelle-Puscian A, Copie-Bergman C, Baia M, Martin-Garcia N, Allory Y, Haioun C. et al. The novel immunosuppressive enzyme IL4I1 is expressed by neoplastic cells of several B-cell lymphomas and by tumor-associated macrophages. Leukemia. 2009;23:952–60.
Chang TY, Li BL, Chang CC, Urano Y. Acyl-coenzyme A:cholesterol acyltransferases. Am J Physiol Endocrinol Metab. 2009;297:E1–9.
Chang TY, Chang CC, Cheng D. Acyl-coenzyme A:cholesterol acyltransferase. Annu Rev Biochem. 1997;66:613–38.
Chang TY, Chang CC, Lin S, Yu C, Li BL, Miyazaki A. Roles of acyl-coenzyme A:cholesterol acyltransferase-1 and -2. Curr Opin Lipido. 2001;12:289–96.
Lu M, Hu XH, Li Q, Xiong Y, Hu GJ, Xu JJ, et al. A specific cholesterol metabolic pathway is established in a subset of HCCs for tumor growth. J Mol Cell Biol. 2013;5:404–15.
Schmidt NM, Wing PAC, Diniz MO, Pallett LJ, Swadling L, Harris JM, et al. Targeting human Acyl-CoA:cholesterol acyltransferase as a dual viral and T cell metabolic checkpoint. Nat Commun. 2021;12:2814.
Yang W, Bai Y, Xiong Y, Zhang J, Chen S, Zheng X, et al. Potentiating the antitumour response of CD8( + ) T cells by modulating cholesterol metabolism. Nature. 2016;531:651–5.
Wang Y, Yang J, Hong T, Chen X, Cui L. SIRT2: controversy and multiple roles in disease and physiology. Ageing Res Rev. 2019;55:100961.
Gomes P, Fleming Outeiro T, Cavadas C. Emerging role of Sirtuin 2 in the regulation of mammalian metabolism. Trends Pharm Sci. 2015;36:756–68.
Chen G, Huang P, Hu C. The role of SIRT2 in cancer: a novel therapeutic target. Int J Cancer. 2020;147:3297–304.
Hamaidi I, Zhang L, Kim N, Wang MH, Iclozan C, Fang B, et al. Sirt2 inhibition enhances metabolic fitness and effector functions of tumor-reactive T cells. Cell Metab. 2020;32:420–36.e412.
Wang B, Ye Y, Yang X, Liu B, Wang Z, Chen S, et al. SIRT2-dependent IDH1 deacetylation inhibits colorectal cancer and liver metastases. EMBO Rep. 2020;21:e48183.
Jing H, Hu J, He B, Negron Abril YL, Stupinski J, Weiser K, et al. A SIRT2-selective inhibitor promotes c-Myc oncoprotein degradation and exhibits broad anticancer activity. Cancer Cell. 2016;29:297–310.
Roshdy E, Mustafa M, Shaltout AE, Radwan MO, Ibrahim MAA, Soliman ME, et al. Selective SIRT2 inhibitors as promising anticancer therapeutics: An update from 2016 to 2020. Eur J Med Chem. 2021;224:113709.
Ducker GS, Rabinowitz JD. One-carbon metabolism in health and disease. Cell Metab. 2017;25:27–42.
Yang L, Garcia Canaveras JC, Chen Z, Wang L, Liang L, Jang C, et al. Serine catabolism feeds NADH when respiration is impaired. Cell Metab. 2020;31:809–21.e806.
Yang M, Vousden KH. Serine and one-carbon metabolism in cancer. Nat Rev Cancer. 2016;16:650–62.
Li G, Wu J, Li L, Jiang P. p53 deficiency induces MTHFD2 transcription to promote cell proliferation and restrain DNA damage. Proc Natl Acad Sci USA. 2021;118:e2019822118.
Shang M, Yang H, Yang R, Chen T, Fu Y, Li Y. et al. The folate cycle enzyme MTHFD2 induces cancer immune evasion through PD-L1 up-regulation. Nat Commun. 2021;12:1940.
Nilsson R, Jain M, Madhusudhan N, Sheppard NG, Strittmatter L, Kampf C, et al. Metabolic enzyme expression highlights a key role for MTHFD2 and the mitochondrial folate pathway in cancer. Nat Commun. 2014;5:3128.
Ben-Sahra I, Hoxhaj G, Ricoult SJH, Asara JM, Manning BD. mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science. 2016;351:728–33.
Sugiura A, Andrejeva G, Voss K, Heintzman DR, Xu X, Madden MZ, et al. MTHFD2 is a metabolic checkpoint controlling effector and regulatory T cell fate and function. Immunity. 2022;55:65–81.e9.
Zhu Z, Leung GKK. More than a metabolic enzyme: MTHFD2 as a novel target for anticancer therapy? Front Oncol. 2020;10:658.
Tedeschi PM, Vazquez A, Kerrigan JE, Bertino JR. Mitochondrial methylenetetrahydrofolate dehydrogenase (MTHFD2) overexpression is associated with tumor cell proliferation and is a novel target for drug development. Mol Cancer Res. 2015;13:1361–6.
Wan X, Wang C, Huang Z, Zhou D, Xiang S, Qi Q, et al. Cisplatin inhibits SIRT3-deacetylation MTHFD2 to disturb cellular redox balance in colorectal cancer cell. Cell Death Dis. 2020;11:649.
Blass E, Ott PA. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat Rev Clin Oncol. 2021;18:215–29.
Goliwas KF, Deshane JS, Elmets CA, Athar M. Moving immune therapy forward targeting TME. Physiol Rev. 2021;101:417–25.
He YF, Mai CT, Pan HD, Liu L, Zhou H, Xie Y. Targeting immunometabolism by active ingredients derived from traditional Chinese medicines for treatment of rheumatoid arthritis. Chin Herb Med. 2021;13:451–60.
Guo Y, Xie YQ, Gao M, Zhao Y, Franco F, Wenes M, et al. Metabolic reprogramming of terminally exhausted CD8( + ) T cells by IL-10 enhances anti-tumor immunity. Nat Immunol. 2021;22:746–56.
Funding
This work was supported by the National Natural Science Foundation of China (No: 82274154), the Tianjin Natural Science Foundation Outstanding Youth Program (No: 23JCJQJC00040), and the Science and Technology Project of Haihe Laboratory of Modern Chinese Medicine (No: 22HHZYSS00008).
Author information
Authors and Affiliations
Contributions
Ranran Su and Yingying Shao equally contributed to writing, reviewing, and editing the manuscript. Manru Huang and Donghui Liu: investigation, resources. Haiyang Yu: supervision, funding acquisition. Yuling Qiu: conceptualization, writing—review and editing, supervision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Su, R., Shao, Y., Huang, M. et al. Immunometabolism in cancer: basic mechanisms and new targeting strategy. Cell Death Discov. 10, 236 (2024). https://doi.org/10.1038/s41420-024-02006-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41420-024-02006-2