Abstract
The biological role of interleukin-37 (IL-37) in cancer is large unknown. Through immunohistochemical detection using 163 primary hepatocellular carcinoma (HCC) clinical specimens, we found the expression of IL-37 was decreased in tumor tissues and the expression level was negatively correlated with tumor size. High expression of IL-37 in HCC tumor tissues was associated with better overall survival (OS) and disease-free survival (DFS). IL-37 expression in tumor tissues was positively associated with the density of tumor-infiltrating CD57+ natural killer (NK) cells, but not with the CD3+ and CD8+ T cells. Consistently, in vitro chemotaxis analysis showed that IL-37- overexpressing HCC cells could recruit more NK cells. The in vivo mouse model experiments also revealed that overexpression IL-37 in HCC cells significantly delayed tumor growth and recruited more NK cells into tumors tissues. Our finding suggested that IL-37 might play an important role for the prognosis of HCC patients via regulating innate immune-action.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors and the third leading cause of cancer-related death worldwide1,2,3,4,5,6. It is the fifth most common cancer in men and the seventh in women and most of the burden is in developing countries2. Despite recent advances in the treatment of HCC, such as hepatic resection, liver transplantation, chemotherapy and tyrosine kinase inhibitors, it remains a highly lethal disease2,5. Most cases of HCC are secondary to chronic hepatitis and cirrhosis resulting from either hepatitis B/C virus infection or from non viral-related causes, such as alcohol or aflatoxin exposure7. The persistent, non-specific and ineffective activation of the immune system within the chronically inflamed liver is thought to promote carcinogenesis7,8,9. Cross talk between HCC tumor cells and their surrounding microenvironments is a key modulator of the processes of hepatocarcinogenesis5. Cancer cells and infiltrating immune cells within the tumor tissues secrete several types of inflammatory cytokines into the tumor microenvironment, which can affect tumor progression and alter the antitumor immune response10,11,12,13. For example, interleukin-6 (IL-6) is a multifunctional inflammatory cytokine produced by Kupffer cells in the liver. High serum IL-6 is associated with a poor prognosis of HCC patients14,15,16,17,18. High expression of interleukin-22 (IL-22) in the HCC microenvironment led to tumor growth, inhibition of apoptosis and promotion of metastasis via STAT3 activation13. Overexpression of intratumoral interleukin-8 (IL-8) correlates with a high frequency of invasion and metastasis in HCC patients10. In contrast, high expression of interleukin-2 (IL-2) in the tumor is a favorable prognostic factor for HCC patients12. Thus, these results suggest that different cytokines might regulate the immuno-microenvironment through different pathways to affect the prognosis of HCC patients.
Interleukin-37 (IL-37, formerly named IL-1F7) is a newly identified member of the interleukin-1 (IL-1) family. The IL-1 family members are proinflammatory cytokines that possess a variety of immunoregulatory properties in response to infection and inflammation19,20,21. For some members, such as IL-1α, IL-1β and IL-18, their receptors, signaling pathways and functions have been studied extensively22. IL-37 is the most-recently discovered member of the IL-1 family not to have a well-defined function23. Transcripts of IL-37 were detected in human tissues, including lung and testis and in colon tumors and human cell lines, such as THP-1, U937 and A43124. Recently, IL-37 emerged as a fundamental anti-inflammatory cytokine, which suppresses innate inflammatory and immune responses22. Nevertheless, Gao et al. found that intratumoral injection of IL-37 resulted in significant growth suppression and the anti-tumor activity was abrogated in nude and SCID mice and in IL-12-, IFN-γ, or Fas ligand-deficient mice, suggested that IL-37 could play an important role in the link between innate and adaptive immunity and may be useful for tumor immunotherapy24. These controversial results indicate a complicated biological role of IL-37 in different environments. Thus, further detailed study is needed to illustrate the potential function of this new IL-1 family member.
In the present study, we investigated the intratumoral expression of IL-37 and its prognostic role in primary HCC. The potential immuno-regulating mechanism of IL-37 in HCC was also explored using an in vitro cell model and in vivo mice experiments. Our results indicated that this recently discovered cytokine might function as an immunological inhibitor of HCC progression via regulation of innate immunity.
Results
Immunohistochemical analysis of IL-37 expression in HCC clinical samples and its relationship with clinicopathological parameters
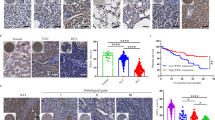
First, the IL-37 expression was investigated in 163 HCC surgical specimens using immunohistochemical staining. Positive staining of IL-37 was mainly detected in the distant normal liver tissues and was located in the cytoplasm of the hepatocytes (Fig. 1A). In cases with adjacent hyperplastic tissue, we often observed positive staining in adjacent non-tumor tissues, but weak staining in tumor tissues (Fig. 1B). In HCC tumor tissues, there was variation in the level of IL-37 expression (Fig. 1C–F). Based on the IL-37 expression levels, HCC patients were divided into two groups, the low group (IL-37- or IL-37 +) and the high group (IL-37++ or IL-37 +++), to investigate the association between the IL-37 expression and the clinical features in HCC patients. As shown in Table 1, IL-37 expression was only positively associated with tumor size (p = 0.028). No correlations were found between the IL-37 expression and age (p = 0.339), gender (p = 0.077), histological differentiation (p = 0.096), liver cirrhosis (p = 0.880), HBV (p = 0.201), serum AFP (p = 0.388), recurrence (p = 0.872) and distant metastasis (p = 0.562).
IL-37 protein expression in primary hepatocellular carcinoma surgical specimens as shown by immunohistochemical detection.
(A) IL-37 expression in distant normal liver tissues. (B) IL-37 expression in the tumor tissues and the adjacent non-tumor tissue. (C) and (D) IL-37 positively staining in some tumor cases. (E) and (F) IL-37 negative staining in some tumor cases. N: non-tumor tissue; T: tumor tissue. B with 100× magnification; A, C–F with 200× magnification).
Correlation between the IL-37 expression and survival of HCC patients
The prognostic value of IL-37 for OS and DFS in HCC patients was evaluated by comparing the patients with high and low IL-37 expressions. Kaplan–Meier curve analysis showed that low IL-37 expression was significantly associated with poor prognosis of HCC patients. The HCC patients with low IL-37 expression had markedly lower OS and DFS rates than those with high IL-37 expression (Fig. 2A and Fig. 2B, p = 0.01 for OS, p = 0.008 for DFS, long rank test, respectively).
The Kaplan-Meier survival analysis of primary HCC patients (n = 163) with high IL-37 expression (n = 63) and low IL-37 expression (n = 100) after surgical resection.
The overall survival (OS) (A) and disease free survival (DFS) (B) rate of the patients in the low-IL-37 group and in the high-IL-37 group.
Univariate and multivariate analyses of prognostic variables in HCC patients
To investigate the effect of IL-37 expression and other clinicopathological parameters on the prognosis (OS) of HCC patients, univariate and multivariate analyses were performed. IL-37 expression, tumor size and histological grade were significant prognostic factors in the univariate analysis (Table 2). Multivariate Cox regression analyses showed that IL-37 was an independent risk factor (p = 0.041, Table 2).
Relationship between IL-37 expression and the level of CD57+ NK cells, CD3+ T cells, as well as CD8+ T cells in the tumor microenvironment
To explore the potential role of IL-37 in the tumor microenvironment, we detected the infiltrating levels of CD57+ NK cells, CD3+ T cells and CD8+ T cells in the same tumor tissues. As shown in Fig. 3A, in tumor tissue with low IL-37 expression, the numbers of CD3+ T cells and CD8+ T cells were comparable with that in the tumor tissue with high IL-37 expression. However, the number of CD57+ NK cells appeared to positively correlate with the intratumoral IL-37 expression level. Comparative analysis further confirmed this tendency, which showed that intratumoral IL-37 expression significantly positively correlated with the number of CD57+ NK cells (Fig. 3B, p = 0.0235). There was no statistically significant correlation between IL-37 expression and the number of CD3+ T cells (Fig. 3C, p = 0.0571) or CD8+ T cells (Fig. 3D, p = 0.0943). Similar to the prognostic role of IL-37 expression, we found that higher levels of infiltrating CD57+NK cells were significantly correlated with a better OS of HCC patients (Fig. 3E, p = 0.002, long rank test).
Relationship between IL-37 expression and CD57+ NK cells, CD3+ T cells and CD8+ T cells in the tumor microenvironment.
(A) Representative photomicrographs showing immunohistochemical staining for IL-37, CD57, CD3 and CD8 in the same primary HCC tumors. The correlation of IL-37 expression with CD57+ NK cells (B), CD3+ T cells (C) and CD8+ T cells (D) was shown. (E) Kaplan-Meier survival curves of HCC patients (n = 163) with high CD57+ NK cells density (n = 82) and low CD57+ NK cells density (n = 81) after surgical resection.
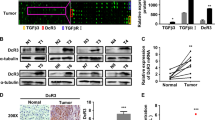
Overexpression IL-37 in human HCC cell line attracts NK cells
To further confirm the relationship between IL-37 expression and level of tumor infiltrating NK cells, we tested the ability of IL-37-overexpressing HCC cells to recruit NK cells using an in vitro chemotaxis assays. We transduced IL-37 into the IL-37 negative human HCC cell line Hep3B using a lentiviral vector (Hep3B/LV-IL37). The same cell line transduced with empty vector was used as a control (Hep3B/LV-NC). The transfection efficiency was verified by western blotting (Fig. 4A and Supplementary Fig. 1). In the transwell chemotaxis assays, the supernatant of Hep3B/LV-IL37 cells recruited a significantly higher number of NK cells compared with the Hep3B/LV-NC cells (Fig. 4B, p = 0.033). There were no differences between the supernatants of Hep3B/LV-IL37 cells and Hep3B/LV-NC cells in terms of recruiting CD3+ T cells or CD8+ T cells (Fig. 4C, p = 0.73; Fig. 4D, p = 0.46).
The impact of IL-37 on the chemotaxis and migration of NK cells, CD3+ T cells and CD8+ T cells.
(A) Stable IL-37 expression in Hep3B cells was confirmed by western blotting. The cropped blots were shown. The recruitment of NK cells (B), CD3+ T cells (C) and CD8+ T cells (D) by the supernatant from Hep3B/LV-IL37 cells or Hep3B/LV-NC cells was compared. The values shown are expressed as the mean ± SD of three independent experiments. The p values were calculated using Student's t-test.
Overexpression of IL-37 in mouse HCC cell line suppresses tumorigenicity and enhances infiltration of CD57+ NK cells into tumor tissues in vivo
To further assess the effects of IL-37 on cancer progression in vivo, we transduced the IL-37 negative mouse HCC cell line Hepa1-6 with exogenous IL-37 using lentiviral vectors (Hepa1-6/LV-IL37). The same cell line transduced with empty vector was used as a control (Hepa1-6/LV-NC). The successful transduction of IL-37 into the Hepa1-6 cells was confirmed by western blotting (Fig. 5A and Supplementary Fig. 1). Hepa1-6/LV-37 or Hepa1-6/LV-NC cells were injected subcutaneously into syngeneic C57BL/6 mice and tumor growth was examined. Compared with the control, the Hepa1-6 cell line overexpressing IL-37 showed significantly delayed tumor growth in the mice (Fig. 5B). The mean tumor volume in the IL-37 overexpressing group (57.99 ± 18.2 mm3) at the end of the observation period was significantly smaller than that of the control group (85.07 ± 26.8 mm3) (Fig. 5B and 5C). Accordingly, the mean tumor weight in the IL-37 overexpressing group (0.064 ± 0.02 g) was also markedly lower than that in the control group (0.174 ± 0.04 g) (Fig. 5D, p = 0.043).
Overexpression of IL-37 delayed tumor growth and enhanced local CD57+ NK cells infiltration within murine tumors.
(A) Stable IL-37 expression in Hepa1-6 cells was confirmed by western blotting. The cropped blots were shown. (B) The tumor growth curves of IL-37 overexpression group (Hepa 1-6/LV-IL37) and control group (Hepa 1-6/LV-NC) were compared. * p < 0.05 versus control. (C) Photographs of dissected tumors from the C57BL/6 mice of IL-37 overexpression group and control group. (D) Dissected tumor weights from IL-37 overexpression group and control group were compared. (E) Representative photomicrographs showing immunohistochemical staining of IL-37, CD57, CD3 and CD8 in murine tumors. (F) The density of CD57+ NK cells, CD3+ or CD8+ T cell infiltration in IL-37 overexpression group and control group was compared. The values shown are expressed as the mean ± SD. The p values were calculated using Student's t-test.
To investigate alterations in the host immune response induced by IL-37 within the tumor microenvironment, we compared the level of tumor infiltrating lymphocytes in the tumor between the IL-37 overexpression group and control group. We first demonstrated that murine CD57+ cells were also murine NK cells, as assessed by immunofluorescence (Supplementary Fig. 2). Similar to the above findings, the number of intratumoral infiltrating CD57+ NK cells was significantly increased in the IL-37 overexpressing group compared with the control group (Fig. 5E and 5F). There was no significant difference for the numbers of intratumoral infiltrating CD3+ T cells and CD8+ T cells between the IL-37 overexpression group and the control group (Fig. 5E and 5F).
To further assess the role of NK cells in the IL-37-mediated antitumor activity, C57BL/6 mice were depleted of NK cells by intraperitoneal injection of anti-Asialo-GM1 antibody before and after Hepa1-6/LV-IL37 inoculation. Mice injection with an isotype antibody at the same dose and schedule were used as controls. Depletion of NK cells impaired the ability of naive mice to reject Hepa1-6/LV-IL37 tumors (Supplementary Fig. 3A). The mean tumor volume in the NK cells-depleted group was 132.76 ± 34.22 mm3 by day 16, compared with 63.67 ± 48.43 mm3 in the control group (Supplementary Fig. 3A and 3B). Accordingly, the mean tumor weight in the NK cells-depleted group (0.138 ± 0.05 g) was also markedly higher than that in the control group (0.059 ± 0.03 g) (Supplementary Fig. 3C, p = 0.017). These data altogether suggested that the anti-tumor activity of IL-37 might be mediated by recruiting CD57+ NK cells to tumor sites in vivo.
Discussion
Accumulating evidence suggests that IL-1 family members play a significant role in tumor immunity. However, different IL-1 family members play different roles in regulating the pro-tumor or anti-tumor effects. For example, IL-1α and IL-1β modulate the microenvironment to the benefit of tumor growth, invasion and metastasis, by activating proteolytic enzymes, stroma formation and angiogenesis25. Conversely, Kuppala et al. showed that IL-18 was an immune-stimulatory cytokine with antitumor activity in preclinical models and could be a useful candidate in gene therapy of lymphoma or lymphoid leukemia26. The physiological roles of IL-37, a newly identified IL-1 family member, in tumors, were unclear.
In the present study, we used a relatively large series of clinical tissue samples to explore intratumoral IL-37 expression in primary HCC and analyze its prognostic value. We found that IL-37 was primarily expressed in normal liver tissues and adjacent non-tumor tissues, but its expression was decreased in tumor tissues. The IL-37 expression level was significantly negatively associated with tumor size, indicating that IL-37 expression in the tumor microenvironment might inhibit tumor growth. Kaplan-Meier survival analysis revealed that high intratumoral IL-37 expression was linked to better OS and DFS in HCC patients and multivariate analyses showed that it is an independent risk factor, indicating that the IL-37 expression level could potentially serve as a valuable prognostic marker for HCC.
To further explore the potential mechanism of IL-37's regulating of HCC progression, we detected tumor infiltrating CD57+ NK cells, CD3+ T cells and CD8+ T cells in the same tumor tissues from HCC patients using serial tissue sections. We found that the intratumor expression level of IL-37 positively correlated with the density of tumor infiltrating CD57+ NK cells, which were significantly associated with better OS rates of HCC patients, indicating that IL-37 might mediate the anti-tumor immunity through regulating NK cell activity in the tumor. The following in vitro transwell chemotaxis assays and in vivo mice experiments provided similar findings to the clinical samples detection. Recent data demonstrate that infiltration of NK cells into tumor sites are required for the induction of potent anti-tumor immune responses27. Bannerji, et al. found that rejection of IL-2-gene modified tumor cells was mediated by NK cells infiltration at the site of inoculation28. Ha, et al. revealed that tumorigenicity of rat hepatoma cells engineered to overexpress IL-12 was decreased in company with an increase of NK cells infiltration29. In prostate cancer, similar results were also observed that NK cells were the merely immune cells to be related to rejection of IL-7 secreting tumor30. Besides, the relationship between high intratumoral levels of NK cells and increased survival has been shown in several types of cancer31. For example, high levels of tumor-infiltrating NK cells have been associated with a significant improvement of clinical outcomes in head and neck squamous carcinoma (HNSCC) patients32. A significant correlation between the density of CD57+ NK cells and a favorable prognosis has been reported in gastric carcinoma and oral squamous carcinoma33. Moreover, Chew et al. also found that the density of NK cells in tumors was correlated positively with tumour apoptosis and negatively with tumor proliferation7. These data, together with ours, suggest that IL-37 might mediate anti-tumor immune responses through recruiting CD57+ NK cells to tumor sites.
In contrast to most studies, which showed that IL-37 is a new anti-inflammatory cytokine, our results indicated that expression of IL-37 in HCC cells would activate local immunity in the microenvironment by regulating the action of NK cells, thus playing a protective role in tumor progression. Similar to our findings, Gao et al. found that treatment of an established MCA205 mouse fibrosarcoma model by single intratumoral injection of Ad-IL-37 resulted in significant growth suppression24. However, they found that the anti-tumor immuno-reaction of IL-37 was dependent on T cells and B cells, but not NKT cells, which is different from our results24. We think this might be a consequence of the type of cancer studied. The detailed mechanism of IL-37's effect on HCC requires further investigation.
In conclusion, our data in the present study show that IL-37 expression is lower in primary HCC tumor tissues and is associated with tumor progress and poor prognosis. Decreasing IL-37 expression in HCC cells might make them lose their ability to recruit NK cells into tumor tissues, leading to a defective anti-tumor immuno-microenvironment. Thus, IL-37 might not only be a valuable prognostic biomarker, but also could be a potential candidate for immuno-gene therapy for HCC.
Methods
Cell lines and culture conditions
The human HCC cell line Hep3B and mice HCC cell line Hepa1-6 (syngeneic to C57BL/6 mice) were obtained from the American Type Culture Collection (Manassas, VA, USA). Hep3B cells were cultured in DMEM (Dulbecco modified Eagle medium) (Gibco, USA) supplemented with 10% heat-inactivated FBS (fetal bovine serum) (Gibco, USA) and 1% penicillin-streptomycin. Hepa1-6 cells were cultured in RPMI 1640 medium (Gibco, USA) supplemented with 10% heat-inactivated FBS and 1% penicillin-streptomycin. All cells were incubated at 37°C in a humidified chamber containing 5% CO2.
Patients and tissue specimens
Formalin-fixed, paraffin-embedded tissues were obtained from 163 HCC patients who underwent surgical resection at the Sun Yat-sen University Cancer Center between 2003 and 2004. None of these patients had received preoperative chemotherapy or radiotherapy. Patients with autoimmune diseases were excluded. The follow-up data from the HCC patients in this study were available and complete. The postoperative follow-up occurred at our outpatient department and included clinical and laboratory examinations every 3 months for the first 2 years, every 6 months during the third to fifth years and annually for an additional 5 years or until patient death, whichever occurred first. Overall survival (OS) was defined as the period from the surgery to death or the last known follow-up. Disease-free survival (DFS) was defined as the period from the surgery to recurrence or the last follow-up if no recurrence was observed. The experiments involving human tissue samples were approved by the Ethics Committee of Sun Yat-sen University Cancer Center and written informed consent was obtained from each patient involved in the study.
Immunohistochemical analysis
The paraffinic-embedded tissue blocks were sectioned at a thickness of 2 μm for immunohistochemistry. The sections were deparaffinized and rehydrated using graded ethanol. For antigen retrieval, the slides were immersed in EDTA (1 mmol/L, pH 8.0) and boiled for 15 minutes in a microwave oven. After rinsing with PBS, endogenous peroxidase was blocked with 3% hydrogen peroxide for 15 minutes at room temperature. The slides were incubated overnight at 4°C with primary monoclonal antibodies, including mouse anti-human IL-37 (Abcam, USA; dilution 1/1000), mouse anti-human CD3 (Zhongshan Golden Bridge Biotech, Beijing, China; dilution 1/100), mouse anti-human CD8 (Zhongshan Golden Bridge Biotech, Beijing, China; dilution 1/100) and rabbit anti-human CD57 (Zhongshan Golden Bridge Biotech, Beijing, China; dilution 1/100). After incubation with the primary antibody, the slides were washed with PBS three times. The sections were incubated with horseradish peroxidase-conjugated secondary antibody (Envision™ Detection Kit, GK500705, Gene Tech) for 30 minutes at room temperature and then washed three more times with PBS. Finally, 3,3′-diaminobenzidine tetrahydrochloride (DAB) was used for signal development and the sections were counterstained with 20% hematoxylin. The slides were dehydrated, cleared and evaluated. Each sample was incubated with an isotypic antibody dilution under the same experimental conditions as the negative control.
Image quantification
The total IL-37 immunostaining score was calculated as both the percentage of positively stained tumor cells and the staining intensity. The percent positivity was scored as “0” (<5%, negative), “1” (5%–25%, sporadic), “2” (25%–50%, focal) or “3” (>50%, diffuse). The staining intensity was scored as “0” (no staining), “1” (weakly stained), “2” (moderately stained) or “3” (strongly stained). The IL-37 immunostaining score was calculated as the percentage positive score × the staining intensity score and ranged from 0 to 9. We defined the IL-37 expression levels as follows: ‘−’ (score 0–1), ‘+’ (score 2–3), ‘++’ (score 4–6) and‘+++’ (score >6). Based on the IL-37 expression levels, the HCC patients were divided into two groups: the low IL-37 expression group (IL-37- or IL-37 +) and the high IL-37 expression group (IL-37++ or IL-37 +++).
The densities of CD57+ NK cells, CD3+ T cells and CD8+ T cells were obtained by manually counting the positively stained cells in ten separate fields under 400× high power magnification. The density of stained cells was determined by calculating the mean number of positively stained cells per high power microscopic field (HPF, 400× magnification). Based on the median number of CD57+ NK cells, CD3+ T cells and CD8+ T cells, the HCC patients were also separated into high and low groups respectively. Two independent observers performed the analysis using a Leica DM IRB inverted research microscope (Leica Microsystems, Wetzlar, Germany).
Overexpression IL-37 in HCC cells
GenePharma Co., Ltd (Shanghai, China) constructed the human IL-37- recombined lentiviral expression vector and the control vector. The LV-IL-37 and LV-control titers were both 1 × 109 TU/ml. The recombinant lentiviruses were stored at −80°C until use. Human HCC cell line Hep3B and mouse HCC cell line Hep1-6 were infected with lentivirus (LV-IL-37 and LV-control) at a multiplicity of infection (MOI) of 30 in the presence of 5 μg/ml polybrene (Sigma, USA). After infection for 48 h, the cells were selected using 5 μg/ml puromycin and screened for stable cell lines (Hep3B/LV-IL37 and Hep3B/LV-NC, Hep1-6/LV-IL37and Hep1-6/LV-NC). Western blotting was used to assess the successful induction of IL-37 expression in all cell types according to our previous methods6. When the western blotting was performed, the gels have been run under the same experimental conditions. The cropped blots were showed.
In vitro chemotaxis assays
Human peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats (Guangzhou Blood Center, Guangzhou, China) by Ficoll-Hypaque gradient centrifugation. NK cells, CD3+ T cells and CD8+ T cells were purified from PBMCs by negative selection using immunomagnetic beads (Invitrogen Dynal AS, Norway). Hep3B/LV-IL37 and Hep3B/LV-NC cells (2 × 105) were plated in six-well culture dishes for 72 h containing 3 ml complete DMEM medium. The supernatant was then collected, centrifuged and stored in aliquots at −80°C.
Chemotaxis assays were performed using polycarbonate filters of 5 μm pore size in 24-well transwell chambers (Corning Incorporated, USA). 5 × 106 purified NK cells, CD3+ T cells and CD8+ T cells were placed to the upper chamber in 100 μl of complete medium and 600 μl culture supernatants from Hep3B/LV-IL37 or Hep3B/LV-NC cells were added to the lower chamber. The cells were incubated at 37°C for 4 h. A 500 μl aliquot of the cells that migrated to the bottom chamber were collected and counted. Scores were calculated as the number of migrated cells versus control. Each experiment was performed in triplicate.
Tumorigenicity assays in C57BL/6 mice
Female C57BL/6 mice (5–6 weeks old) were obtained from the Medical Experimental Animal Center of Guangdong Province. Mice were maintained in a specific pathogen-free environment and all animal procedures were conducted in compliance with the guidelines of the laboratory animal ethics committee of Sun Yat-sen University. Hepa1-6/LV-IL37 and Hepa1-6/LV-NC cells were harvested and injected subcutaneously into the posterior flank of mice (5 × 106 tumor cells in 100 μl of PBS). The tumor size was monitored every 3 days by measuring the length (L) and width (W) of the tumor with calipers. The tumor volume was calculated according to the following formula: (L × W2)/2. All the mice were sacrificed 16 days after inoculation and the tumors were harvested and photographed. The tumors were then cut into 2 mm3 cubes, fixed in 10% formalin and embedded in paraffin. Sections were cut at 2 μm thickness and mounted onto poly-L-lysine coated microscope slides for subsequent immunohistochemical analysis.
CD57+, CD3+ and CD8+ positive cells were detected using rabbit anti-mouse CD57 polyclonal antibody (1:400 dilution; Novus Biologicals, USA), rabbit anti-mouse CD3γ monoclonal antibody (1:500 dilution; Epitomics, USA) or rabbit anti-mouse CD8A polyclonal antibody (1:300 dilution; Abnova, USA), respectively. Some specimens were simultaneously incubated with Alexa Flour 488 anti-mouse NK-1.1 monoclonal antibody (1:100 dilution; Biolegend, Beijing, China) and rabbit anti-mouse CD57 polyclonal antibody (1:100 dilution), followed by Alexa Fluor 594 donkey anti-mouse IgG (H + L) (10 μg/ml; Invitrogen, Shanghai, China). CD57+ NK cells, CD3+ T cells and CD8+ T cells were quantified as the mean number of positively stained cells per random high power microscopic field (HPF, 400× magnification) from ten fields per section.
Mice deleted NK cells by in vivo antibody treatment were used to evaluate the relative contribution of NK cells to IL-37-mediated antitumor activity. C57BL/6 mice were injected subcutaneously with 5 × 106 Hepa1-6/LV-IL37 cells on day 0. NK cells were deleted by intraperitoneal injection of 50 μg of rabbit anti-Asialo-GM1 polyclonal antibody (eBioscience, San Diego, CA, USA) on day -2 and then with 50 μg of the same antibody on day 0, 3 and 7. Mice injected intraperitoneal injection with an isotype antibody at the same dose and schedule were used as controls. Tumor growth and volume calculation were monitored as described above.
Statistical analysis
Quantitative values were expressed as the mean ± SD or median (range). The IL-37 immunostaining score or median value of CD57+ NK cells was used as a cut-off for the subgroups of all immunohistochemical variables in our data. Chi-squared tests were used to assess the relationship between IL-37 expression and the clinicopathological features. OS and DFS curves were calculated according to the Kaplan-Meier method and were analyzed by the log-rank test. Prognostic factors were examined by univariate and multivariate analyses using a Cox proportional hazards model. The differences in the densities of CD57+ NK cells, CD3+ T cells and CD8+ T cells between the IL-37 high group and low group were determined by unpaired t test with Welch's correction. A two-sided p-value <0.05 was considered statistically significant. All statistical analyses were performed using SPSS software (version 16.0; SPSS Inc., Chicago, IL, USA).
References
Giannelli, G. & Antonaci, S. Novel concepts in hepatocellular carcinoma: from molecular research to clinical practice. J Clin Gastroenterol. 40, 842–846 (2006).
Guo, C. L. et al. Associations between infiltrating lymphocyte subsets and hepatocellular carcinoma. Asian Pac J Cancer Prev. 13, 5909–5913 (2012).
Murray, C. J. & Lopez, A. D. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 349, 1269–1276 (1997).
Thomas, M. B. & Zhu, A. X. Hepatocellular carcinoma: the need for progress. J Clin Oncol. 23, 2892–2899 (2005).
Yang, J. D., Nakamura, I. & Roberts, L. R. The tumor microenvironment in hepatocellular carcinoma: current status and therapeutic targets. Semin Cancer Biol. 21, 35–43 (2011).
Zhao, J. J. et al. Identification of LZAP as a new candidate tumor suppressor in hepatocellular carcinoma. PLoS One. 6, e26608 (2011).
Chew, V. et al. Inflammatory tumour microenvironment is associated with superior survival in hepatocellular carcinoma patients. J Hepatol. 52, 370–379 (2010).
Kremsdorf, D., Soussan, P., Paterlini-Brechot, P. & Brechot, C. Hepatitis B virus-related hepatocellular carcinoma: paradigms for viral-related human carcinogenesis. Oncogene. 25, 3823–3833 (2006).
Nakamoto, Y., Guidotti, L. G., Kuhlen, C. V., Fowler, P. & Chisari, F. V. Immune pathogenesis of hepatocellular carcinoma. J Exp Med. 188, 341–350 (1998).
Akiba, J., Yano, H., Ogasawara, S., Higaki, K. & Kojiro, M. Expression and function of interleukin-8 in human hepatocellular carcinoma. Int J Oncol. 18, 257–264 (2001).
Hu, P. et al. Expression of interleukins-23 and 27 leads to successful gene therapy of hepatocellular carcinoma. Mol Immunol. 46, 1654–1662 (2009).
Ikeguchi, M. & Hirooka, Y. Interleukin-2 gene expression is a new biological prognostic marker in hepatocellular carcinomas. Onkologie. 28, 255–259 (2005).
Jiang, R. et al. Interleukin-22 promotes human hepatocellular carcinoma by activation of STAT3. Hepatology. 54, 900–909 (2011).
Cressman, D. E. et al. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 274, 1379–1383 (1996).
Nakagawa, H. et al. Serum IL-6 levels and the risk for hepatocarcinogenesis in chronic hepatitis C patients: an analysis based on gender differences. Int J Cancer. 125, 2264–2269 (2009).
Tilg, H. et al. Serum levels of cytokines in chronic liver diseases. Gastroenterology. 103, 264–274 (1992).
Wong, V. W. et al. High serum interleukin-6 level predicts future hepatocellular carcinoma development in patients with chronic hepatitis B. Int J Cancer. 124, 2766–2770 (2009).
Zhu, A. X. et al. Efficacy, safety and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study. J Clin Oncol. 27, 3027–3035 (2009).
Bulau, A. M. et al. In vivo expression of interleukin-37 reduces local and systemic inflammation in concanavalin A-induced hepatitis. ScientificWorldJournal. 11, 2480–2490 (2011).
Dinarello, C. A. Biologic basis for interleukin-1 in disease. Blood. 87, 2095–2147 (1996).
Dinarello, C. A. Interleukin-1, interleukin-1 receptors and interleukin-1 receptor antagonist. Int Rev Immunol. 16, 457–499 (1998).
Nold, M. F. et al. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol. 11, 1014–1022 (2010).
McNamee, E. N. et al. Interleukin 37 expression protects mice from colitis. Proc Natl Acad Sci U S A. 108, 16711–16716 (2011).
Gao, W. et al. Innate immunity mediated by the cytokine IL-1 homologue 4 (IL-1H4/IL-1F7) induces IL-12-dependent adaptive and profound antitumor immunity. J Immunol. 170, 107–113 (2003).
Apte, R. N. & Voronov, E. Interleukin-1--a major pleiotropic cytokine in tumor-host interactions. Semin Cancer Biol. 12, 277–290 (2002).
Kuppala, M. B. et al. Immunotherapeutic approach for better management of cancer--role of IL-18. Asian Pac J Cancer Prev. 13, 5353–5361 (2012).
Moretta, L. et al. Human NK Cells: From Surface Receptors to the Therapy of Leukemias and Solid Tumors. Front Immunol. 5, 87 (2014).
Bannerji, R., Arroyo, C. D., Cordon-Cardo, C. & Gilboa, E. The role of IL-2 secreted from genetically modified tumor cells in the establishment of antitumor immunity. J Immunol. 152, 2324–2332 (1994).
Ha, S. J., Lee, S. B., Kim, C. M., Shin, H. S. & Sung, Y. C. Rapid recruitment of macrophages in interleukin-12-mediated tumour regression. Immunology. 95, 156–163 (1998).
Schroten, C. et al. Tumor protection by IL-7 secreting whole cell vaccine is merely mediated by NK1.1-positive cells. J Immunother. 35, 125–130 (2012).
Senovilla, L. et al. Trial watch: Prognostic and predictive value of the immune infiltrate in cancer. Oncoimmunology. 1, 1323–1343 (2012).
van Herpen, C. M. et al. Intratumoral recombinant human interleukin-12 administration in head and neck squamous cell carcinoma patients modifies locoregional lymph node architecture and induces natural killer cell infiltration in the primary tumor. Clin Cancer Res. 11, 1899–1909 (2005).
Baginska, J. et al. The Critical Role of the Tumor Microenvironment in Shaping Natural Killer Cell-Mediated Anti-Tumor Immunity. Front Immunol. 4, 490 (2013).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31270964), Natural Science Foundation of Guangdong Province, China (S2013020012722) and Scientific and Technological Plan Project of Guangdong Province, China (2011A030400004).
Author information
Authors and Affiliations
Contributions
J.J.Z., K.P. and Q.Z.P. designed and performed most experiments and data analysis. Q.Z.P., D.S.W. and Q.J.W. carried out the Immunohistochemical detection and analysis. L.L., D.D.W. and H.X.Z. contributed the IL-37 gene transfection and stable cell lines establishing. S.S.J., J.J.L. and X.F.Z. contributed the in vitro chemotaxis analysis and in vivo mice experiments. J.C.X. designed and directed the overall project. J.J.Z., K.P. and J.C.X. wrote the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary figures
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. The images in this article are included in the article's Creative Commons license, unless indicated otherwise in the image credit; if the image is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the image. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Zhao, JJ., Pan, QZ., Pan, K. et al. Interleukin-37 Mediates the Antitumor Activity in Hepatocellular Carcinoma: Role for CD57+ NK Cells. Sci Rep 4, 5177 (2014). https://doi.org/10.1038/srep05177
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep05177
This article is cited by
-
Interleukin-37 is involved in the immunopathogenesis of infectious mononucleosis
Italian Journal of Pediatrics (2023)
-
IL-1R8 expression in DLBCL regulates NK cell recruitment and influences patient prognosis
Functional & Integrative Genomics (2023)
-
Association between IL-37 gene polymorphisms and risk of HBV-related liver disease in a Saudi Arabian population
Scientific Reports (2019)
-
Intracellular mature IL-37 suppresses tumor metastasis via inhibiting Rac1 activation
Oncogene (2018)
-
Reduced IL-37 Production Increases Spontaneous Chemokine Expressions in Colon Epithelial Cells
Digestive Diseases and Sciences (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.