Our memories depend on our ability to recall details about the world—a child’s face, a goose, a lake. To transform them into actual experiences, though, the brain must somehow merge these individual elements into an integrated whole—the look on that child’s face when she sees a flock of geese suddenly take flight from a lakeside stand of reeds.
A cohesive sense of memory relies on other factors, too. Our survival over the millennia has depended on recalling not only the right information—say, a lion or a snake—but also the context. Did we encounter the animal during a surprise confrontation on an isolated stretch of African savanna or as part of an unhurried viewing at the San Diego Zoo?
To steer clear of other kinds of predators in our daily lives, we also need to be able to link memories over time: Judging whether a seemingly attractive investment is worth pursuing depends on the source of a recommendation—the probity, for instance, of the person who suggested it. Failing to connect the two can have disastrous consequences.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
The field of neuroscience is starting to grapple with how the brain links memories across space and time. Until now, the vast majority of studies have focused on the way we acquire, store, recall and alter individual memories. Most memories, though, do not exist on their own as single, isolated entities. Instead one recollection summons the next, establishing intricate sequences of memories that help us to better predict and comprehend the world around us.
The fundamental mechanisms the brain uses to create these linked memories are beginning to reveal themselves—after 20 years of research in my laboratory and others. Understanding the physical processes involved in interweaving individual memories will do more than just provide insight into how the brain works. It may help us to prevent memory disorders that disrupt our ability to create and tie together memories.
A Happy Accident
When we began our studies of memory linking in the late 1990s, we lacked the tools and basic knowledge we needed to tackle this subject. A key first step in determining how memories are intertwined was our discovery of a concept called memory allocation, the realization that the brain uses specific rules to assign bits of learned information to discrete groups of neurons in regions of the brain involved in forming the memory.
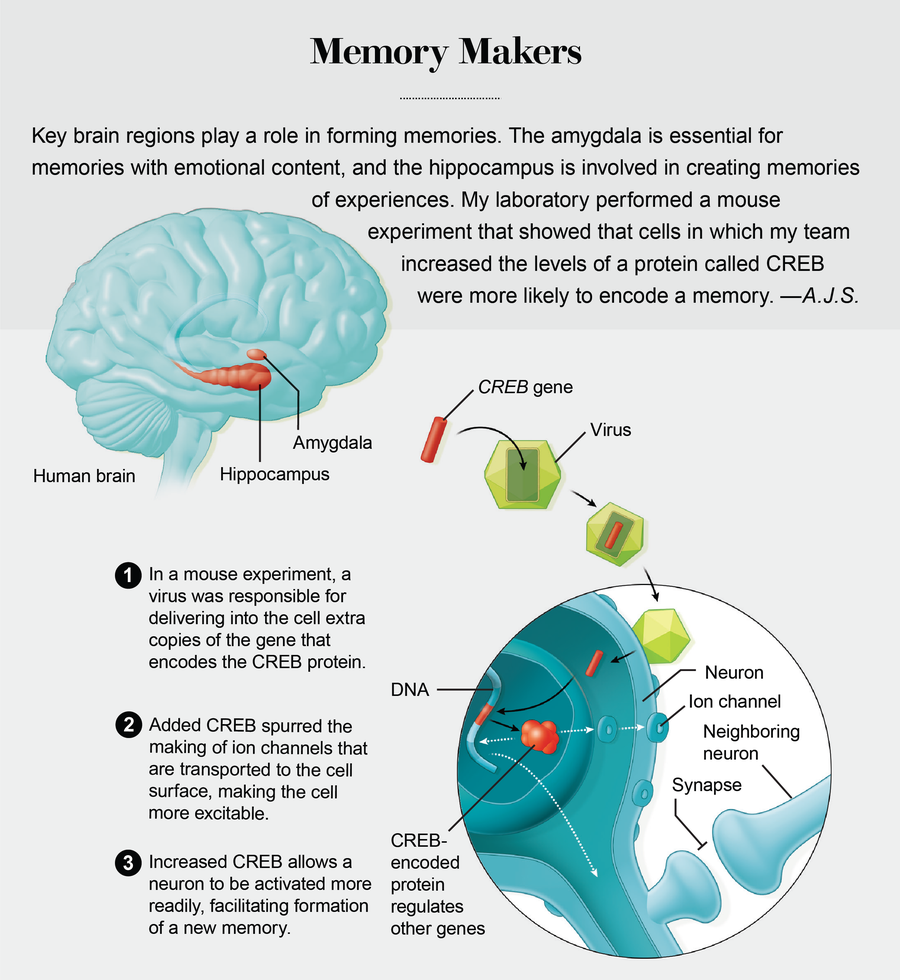
Serendipity played a key role in the discovery of memory allocation. It started with a conversation I had with Michael Davis, a friend and colleague now at Emory University, during a visit to Yale University in 1998. Davis shared with me the findings of studies in which his lab manipulated a gene known as CREB to enhance emotional memory in rats—the association, for example, between a tone and an electric shock. Previously, my lab (now at the University of California, Los Angeles) and other researchers had shown that the CREB gene was needed to form long-term memories. The CREB gene accomplishes this task by encoding a protein that regulates the expression of other genes needed for memory. During learning, some synapses (the cellular structures neurons use to communicate) are built up, or strengthened, so that they can facilitate interaction among cells. The CREB protein acts as a molecular architect of this process. Without its help, most experiences would be forgotten.
What surprised me was that Davis’s group was able to improve memory, even though his lab increased CREB levels in only a subset of the overall population of neurons of the amygdala, a brain region critical for emotional memory. The question that lingered with me for months after my visit to Yale was, How did the memory end up in just the few cells where it could take advantage of the higher CREB levels? Could it be that CREB not only orchestrated memory formation but also helped to ensure that cells with CREB were more likely to be involved in memory formation? In our own investigations of CREB, we homed in on its function within specific brain regions we knew were involved with memory: the amygdala and the hippocampus. The latter stores an internal map of one’s surroundings.

MICROSCOPE mounted on the head of a live mouse lets researchers inspect the activity of brain cells where memories are stored. Credit: Denise J. Cai Integrative Center for Learning and Memory, University of California, Los Angeles
Science is just as much about finding questions as it is about answering them. What that conversation with Davis helped me realize is that neuroscientists knew very little about the rules, if, indeed, there were any, of how a given memory is allocated to the neurons in each of the brain regions that process and store our recollections. So we decided to look more closely.
Our first big break came after we recruited neuroscientist Sheena A. Josselyn, who had studied CREB in Davis’s lab. In a series of animal experiments that she conducted in my lab and later with colleagues at her own lab at the University of Toronto, Josselyn used a virus to introduce extra copies of CREB into specific neurons within the mouse amygdala. She showed that those neurons were nearly four times more likely to store a fearful memory than neighboring ones.
In 2007, after almost a decade of effort, my lab, in collaboration with Josselyn’s team, finally published evidence that emotional memories are not randomly assigned to neurons within the amygdala. Rather the cells tapped to store these memories are those that have more of the CREB protein. Just as important, subsequent experiments showed that CREB has a similar function in other brain regions, including the hippocampus and the cortex, the outermost layer.
Switching Memories On and Off
To confirm CREB’s role in memory allocation, we turned to newly developed methods that have transformed the study of memory in recent years. These lab techniques make it possible to either activate or switch off neurons—in effect, eliciting or silencing a memory.
As one example, Yu Zhou, then in my lab, genetically modified a small set of mouse amygdala neurons so that they had higher CREB levels and expressed another protein engineered by Edward Callaway’s lab at the Salk Institute for Biological Studies in La Jolla, Calif. Callaway’s nifty protein allowed us to silence the CREB neurons at a time of our choosing. When we shut off the neurons that had high CREB, leaving their counterparts with lower levels of the protein still active, emotional memory was suppressed, a result that provides evidence that neurons with higher levels of CREB are more likely to be involved in memory storage.
We knew that higher levels of CREB could determine which cells stored a given memory, but we did not know how this happened. Robert Malenka of Stanford University and his colleagues had discovered that increasing CREB in certain neurons meant they were more easily activated. Could this increase in excitability be the reason why neurons with higher CREB levels were picked for memory storage?
To address that question, Zhou modified amygdala neurons to produce more CREB. Using tiny microelectrodes, she determined how easily these neurons are activated, a measure of excitability. The results confirmed that the modified neurons were more easily switched on, compared with their unaltered counterparts. The elevated excitability (an enhanced readiness to receive and pass on electrical impulses that carry information between neurons) suggested that the cells may have been better prepared to initiate the set of processes needed for laying down a memory.
To test that idea, Zhou also looked at synaptic connections involving the neurons with more CREB. A considerable body of evidence has shown that increases in the strength of synaptic connections are critical for memory formation. After training the mice on a task that subsequently evoked emotional memories, she tested the strength of synaptic connections of the amygdala neurons with higher CREB levels to see whether they had stronger connections, compared with cells that had not been altered to produce more CREB.
To do this, she stimulated the synapses of these cells with a small electric current and recorded their responses with tiny electrodes embedded within the cells. As expected, the amygdala neurons with higher CREB had stronger synapses than other cells, a result consistent with the idea that they were more likely to have stored the emotional memory.
In still more recent work, Josselyn’s lab demonstrated that a memory of a fearful experience could be stored in a predetermined set of amygdala neurons by genetically engineering them with a specific type of ion channel that increases the excitability of these neurons. Ion channels form pores on the surface of the cells, and the particular ones that Josselyn chose allowed these cells to be more easily activated. Similarly, neuroscientist Albert Lee’s lab at the Howard Hughes Medical Institute’s Janelia Research Campus in Ashburn, Va., reported that artificially increasing the excitability of hippocampal neurons in a specific place while animals ran around a track made those neurons more likely to respond to that location in the track, a result consistent with our findings that excitability has a critical role in determining which cells are engaged in storing a given memory.
Finally, our group, as well as Josselyn’s, took advantage of a groundbreaking technology called optogenetics that uses light to either activate or inhibit neurons. We used the technique to switch on specific neurons that had higher CREB levels. Thomas Rogerson and Balaji Jayaprakash, both then in my lab, began by engineering amygdala neurons to produce more CREB and channelrhodopsin 2 (ChR2), an ion channel activated by blue light. We then showed that we could artificially trigger recall of a fear memory in mice when we used the light to turn on amygdala neurons with higher CREB but not ones with lesser levels of the protein, confirming that the memory was stored in those neurons.
Linking Up
In 2009 I was asked to write an article on our memory research, and I took that opportunity to introduce our ideas on how memories are linked over time. CREB’s ability to regulate which cells form a given memory—in other words, memory allocation—led me to the hypothesis that this process may be key to the ability to connect separate memories, what my lab now calls the “allocate to link” hypothesis. Because memory allocation occurs in a subset of neurons having higher CREB that are more easily activated, this process primes these neurons to readily store another memory. When two memories share many of the same neurons, they are formally linked.
Consequently, activation of those neurons during recall of one of the two memories triggers recall of the other. Key to this idea was the prediction that two memories formed closer in time—both within a day—are more likely to be linked than when they are separated by longer periods. With intervals much longer than a day, the second memory no longer benefits from the excitability triggered by the first memory and so is stored in a different population of neurons. The time-limited nature of memory linking makes sense because events that occur within the span of a day are far more likely to be relevant to one another than those separated by, say, a week.
Writing the article and outlining these ideas drew me even more to the challenge of how we might test them. The allocate-to-link hypothesis was straightforward, but it was not at all clear how we would confirm its legitimacy. Testing had to wait for the right time.
The situation changed when Denise J. Cai and Justin Shobe, both then in my lab, joined the project. Cai came up with a clever idea. Together with Shobe, she exposed mice to two chambers during the same day within an interval of five hours, hoping that the memories of the two chambers would be linked. Later she gave them a mild paw shock in the second chamber. As expected, when she subsequently placed the mice in the chamber where they received the shock, they froze, presumably because they remembered that they had received a shock there. Mice freeze as a natural reaction to fear because most predators notice prey better when they move.
The critical result emerged when Cai and Shobe placed the mice in the neutral chamber. We reasoned that if the memories from both chambers were linked, the mice in the neutral space would be reminded of being shocked in the other chamber and thus would freeze in anticipation—and that is exactly what we found.
We also guessed that the two memories would be less likely to be linked if they were separated by a seven-day interval. And indeed, reexposing the animals to the neutral chamber after the longer time span did not remind them of the shock chamber, and they did not freeze. In general, with time intervals much longer than a day, memories remain unlinked. Remarkably, studies in Jill Leutgeb’s lab at the University of California, San Diego, suggested that the very brain region in our experiments has the ability to distinguish between time intervals within the time frames we had tested (many hours), a result that was in agreement with our findings.
These behavioral findings were exciting, but they did not test a key prediction of the hypothesis—that discrete memories formed at closely spaced intervals are stored in the same brain area in overlapping populations of neurons. This physical overlap links the two memories, so that the recall of one brings to mind the other.
Visualizing Memories
To really test the allocate-to-link hypothesis would require nothing short of being able to see memories in the brain as they were being created. Techniques for imaging neurons in live mice are already in use, but they all required that the heads of the mice be fixed to large microscopes, a setup not conducive to the behavioral experiments needed to test the hypothesis.
I find it amazing, though, how many times in my career the right technique has come along just when we need it the most. I happened to attend a seminar at U.C.L.A., given by Mark Schnitzer of Stanford, that described a tiny microscope his lab had just invented that could visualize the activity of neurons in freely moving mice. This two- to three-gram microscope can be mounted like a hat on an animal’s head. The instrument was what our group needed to track the neurons activated by a given memory. Schnitzer and his colleagues had shown that it could be used to track the very neurons we were studying across time. Therefore, we could use their revolutionary miniature microscopes to test a key prediction of our allocate-to-link hypothesis: many of the neurons active during one memory become active again a few hours later during the creation of another linked memory.
We were so excited by the promise of this wonderful invention that we decided to engineer our own version of the microscope. We teamed up with Peyman Golshani’s and Baljit Khakh’s labs, both at U.C.L.A., and together we hired a talented postdoctoral fellow, Daniel Aharoni, who went on to engineer what we came to call the U.C.L.A. miniscopes. Similar to the Schnitzer microscopes, our miniscopes are equipped with a lens that could be embedded near the brain cells we wanted to record from. The device is snapped onto a base plate secured to the animal’s skull, holding it stable during training tasks and memory testing. Just as we borrowed techniques from other researchers, we were also glad to share. We are avid supporters of the open-source movement in science and have made our designs and software for the U.C.L.A. miniscopes available to hundreds of other groups worldwide.
To visualize the activity of neurons with the miniscopes, Cai and her colleague Tristan Shuman took advantage of an imaging technique that genetically engineers neurons in an animal so that they fluoresce when calcium levels in the cells rise—it is known as a genetically encoded calcium indicator.
We decided to focus on the CA1 region of the hippocampus because of its role in learning and remembering places, such as the chambers that we had used in our behavioral experiments. The mice wearing their miniscope hats were placed in the two chambers. We wanted to know whether the time interval between exposures to the different chambers affected which neurons were activated.
Credit: Tami Tolpa
The results were more than we had expected! Essentially our miniscope and behavioral experiments showed that when the mice linked the memories of the two chambers, many of the CA1 neurons that became active when the animals visited the first chamber were also switched on when they explored in the second chamber. If the interval between visits was about five hours, the mice formed two memories in a similar cluster of neurons. When the time lapse increased to seven days, this overlapping pattern of activation did not appear.
We were delighted by this finding because it confirmed a basic premise of the allocate-to-link hypothesis: memories couple when they are stored in overlapping populations of neurons. If you later reactivate an ensemble of neurons formed for either of two memories, it stimulates the other one and facilitates its recall.
Tagging Memories
To further validate the miniscope results, Cai turned to another method developed by neuroscientist Mark Mayford, now at the Scripps Research Institute. This experiment involved Mayford’s technique, called the TetTag system (for tetracycline tag). When a memory is formed during a transgenic mouse’s visit to a chamber, TetTag marks activated neurons with a fluorescent marker that remains intact for weeks.
Postmortem studies of the animals can then compare the recently activated neurons—tagged by genes that are expressed immediately after memory formation—with those marked by the long-lasting tag. This step identifies not only neurons switched on by one event—in which case a neuron features a single fluorescing tag—but also those activated by two occurrences: the glowing of both tags.
Using the same experimental setup as before, Cai and her team showed that during a short, five-hour interval, the overlap between the neurons encoding each of two memories with double tags was significantly greater than would be expected by chance. For a seven-day interval, the overlap between two experiences was not significantly above the level of chance.
Other experiments by Josselyn’s Toronto team provided still more evidence of the validity of our memory-linking hypothesis. Not only did her group carry out a different version of the neuronal tagging experiment, the scientists also found independent behavioral evidence for memory linking. The Toronto researchers reasoned that if populations of neurons encoding two memories overlapped, increases in CREB levels triggered by the first memory would also strengthen a second memory. But instead of exposing the mice to different places, as in our work, Josselyn’s team trained the animals to learn to recognize two different tones. Training on the first tone strengthened the memory of a second tone if the two training sessions occurred within six hours but not from six to 24 hours.
Recently Kaoru Inokuchi and his colleagues at the University of Toyama in Japan took this analysis a step further. They used optogenetics to inactivate the group of cells that was shared by two different emotional memories while leaving other cells undisturbed, including those that were unique to each of the two memories. The investigators showed that by inactivating the shared cells, they were able to disrupt the linking between the two memories without affecting recall of each individual memory. This elegant experiment provided direct evidence that the neurons shared by two memories are key for memory linking. It also added to the number of labs that provided independent evidence for our fledgling allocate-to-link hypothesis.
Improving Memory in Aging
Next, we decided to study memory linking in older mice. Compared with young mice, older mice have lower levels of CREB in the brain, including in neurons in the CA1 area of the hippocampus, and consequently lower excitability. Knowing that, we predicted that aging mice should run into difficulties in linking memories. So Cai and her colleagues set about repeating many of the same experiments we had already completed in older animals. The results surprised us. Experienced scientists know that hypotheses are just tools. We do not expect them to be necessarily right. Inevitable failures help us to reshape our ideas along the way. But this time, our hunches proved correct.
.png?w=900)
Credit: Tami Tolpa (illustrations); Source: “A Shared Neural Ensemble Links Distinct Contextual Memories Encoded Close in Time,” by Denise J. Cai et al., in Nature, Vol. 534; June 2, 2016 (neuron images)
I still remember when Cai burst into my office, slightly out of breath. She told me that the middle-aged mice, despite remembering each individual chamber, indeed had problems linking the memories, even when they were exposed to them five hours apart, an interval that presented no difficulty for younger mice. Compared with the young adult mice, the miniscope imaging of the older animals revealed a lack of overlap between stored memories.
We were both excited but also skeptical, so we went right back and repeated the experiments. The second time around, the results became only more convincing. The neurons in middle-aged mice with lower CREB levels did not link memories as easily as those in young mice.
These results emboldened us to broaden the scope of our investigation. Could we increase artificially the excitability of a subset of CA1 neurons just when the older mice explored the two chambers, ensuring that some of the CA1 neurons activated in one chamber were also switched on when the animals moved to the second?
To accomplish this, we took advantage of a groundbreaking method that genetically engineers receptors onto the surface of a cell, which allows control over the cell’s function. The technique bears the memorable techie acronym DREADD (for designer receptors exclusively activated by designer drugs). Activating the DREADD receptors allowed us to turn on the same subset of CA1 neurons while the animals explored both chambers, thereby forging a link between their memories of the two enclosures.
I must confess that at first the idea for this experiment sounded preposterous. There are any number of reasons why it could have failed. For one thing, memories for places involve many millions of neurons spread throughout multiple interconnected brain regions, not just the CA1 region. Aging could have affected memory-linking processes in many, if not all, of these areas. Thus, even if we were successful in increasing excitability in a subset of CA1 neurons, these cells may not have been the right ones. What is more, we may not have triggered the right levels of excitability.
But the experiment worked. The key for this type of Hail Mary trial is to balance investment in time and money with the potential payoff that may be forthcoming. Nevertheless, in this case, I can safely say that luck was on our side. By restoring increases in excitability in a specific subset of CA1 neurons of middle-aged mice, we were able to allocate the two memories to many of the same CA1 neurons and thus restore memory linking in these middle-aged mice.
Research from other labs in both rodents and humans has also elucidated how one memory can be intertwined with another. The late neuroscientist Howard Eichenbaum of Boston University demonstrated that rats are capable of finding connections between memories that share content. Neuroscientist Alison Preston of the University of Texas at Austin and her colleagues showed that when memories share content, humans can link them more easily. Recalling one will likely bring back the other. In 2017 the same group also showed that temporal proximity facilitates the integration of memories across time, a result in humans with striking parallels to our results in mice.
The growing arsenal of tools at our disposal to measure and control neural activity is beginning to unravel the mechanisms our brain uses to organize information. Our team is now trying to extend this work in new ways. Together with computational neuroscientist Panayiota Poirazi of the Institute of Molecular Biology and Biotechnology at the Foundation for Research and Technology-Hellas in Greece, we are building computer models to simulate how and when memories link up. We are also trying to figure out the mechanisms that control the time intervals needed for memory linking in different brain structures.
So far a number of broad-ranging experiments carried out by multiple labs all strongly support the allocate-to-link hypothesis. We hope that an understanding of how memories become entangled may help us to develop treatments for memory problems that are common across a wide swath of psychiatric conditions, from age-related cognitive decline to schizophrenia, depression and bipolar disorder. Beyond clinical implications, the studies we have described reflect an exciting new era in memory research in which the experiments we do are no longer limited by the techniques we have at our disposal but by the reaches of our imagination.

