Abstract
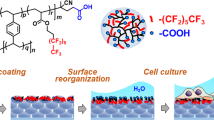
The attachment of poly(N-isopropylacrylamide) (PNIPAM) onto glass slides by using a hyperbranched poly(siloxysilane) (HBPS) as a linker was investigated to develop a thermoresponsive glass surface for cell sheet engineering. A reversible addition–fragmentation chain transfer (RAFT) polymerization chain transfer agent (CTA) was introduced onto the termini of the HBPS via the polycondensation of an AB2 monomer and subsequent suitable end-group modification. Living radical polymerization of NIPAM from the CTA-termini of HBPS was achieved, yielding a copolymer of HBPS and PNIPAM (HBPS-g-PNIPAM). The glass slides were PNIPAM-coated by drop casting of HBPS-g-PNIPAM in tetrahydrofuran (THF), and immobilization was confirmed by X-ray photoelectron spectroscopy, atomic force microscopy and contact angle measurements. The resulting glass slide was applied to cell culture of mouse 3T3 fibroblasts, and attachment and proliferation were successfully achieved at 37 °C after 2 or 4 days. Reducing the temperature to 20 °C for 15 min permitted the detachment of the cell sheet. The glass slide was successfully reused for cell culture, thus confirming the strong attachment of the polymer onto the surface.
Similar content being viewed by others
Introduction
Regenerative medicine is one of the most promising medicines for treating patients with diseases that are difficult to treat and physically lessen function, in contrast to symptomatic treatment medicine that is limited to reducing the effects of the disease on the body. This approach is based on using cells or tissues as medicine rather than molecularly targeted drugs. The transplantation of single cells as a suspension is not efficient because many cells are rapidly lost after transplantation, thus limiting the results. To overcome this problem, cell sheets or tissues have been investigated and found to exhibit promising results.1, 2 However, detachment of the resulting sheets from their growth support without damaging them was difficult until Okano and colleagues1, 2, 3, 4, 5 introduced the thermoresponsive surface for cell culture as a robust method for attachment of the cells, followed by detachment of the sheets. This methodology is based on the hydrophilic–hydrophobic changes in some polymers after exposure to an external trigger (for example, pH and temperature), and these materials are commonly referred to as smart polymers. For example, Okano and colleagues1, 2, 3, 4, 5 have introduced poly(N-isopropylacrylamide), one of the most well-known smart polymers, owing to a drastic change in its hydrophilicity resulting from a lower critical solution temperature (LCST) of 32 °C near body temperature,6 onto the surface of polymeric dishes. Above the LCST, the hydrophobic surface permits cell culture, and through reducing the temperature below the LCST, the cell sheets spontaneously detach from the surface.
Introduction of a thermoresponsive polymer onto a surface for cell culture is currently limited to the modification method that typically requires expensive machinery, such as an electron beam for the polymeric surface, or tedious surface modification of the glass slide surface7, 8, 9 that requires several surface treatments. In the latter technique, many steps are required to introduce a thermoresponsive property onto the surface as follows: (1) pretreatment of the glass with a plasma cleaner or acid solution, (2) silane coupling modification to introduce functional groups, (3) immobilization of the initiator on the surface and (4) radical polymerization of NIPAM.
However, our group has recently developed a ‘grafting on’ method using a hyperbranched poly(styrene) (PSt) as a link between the surface of the PSt dish and the poly(N-isopropylacrylamide) (PNIPAM) segment.10 Despite producing results comparable to the electron beam-modified surface, this system has been limited to polymer surface modification because of the absence of strong adhesion between PSt and the nonpolymer surface. For other applications, glass surface modifications with linear siloxane-based thermoresponsive copolymers have been reported but have not been tested for cell culture.11, 12, 13 In our group, we have developed a hybrid inorganic–organic hyperbranched poly(siloxysilane) (HBPS) that efficiently attaches onto many different surfaces, such as silicon wafers,14 silica beads15 or aluminum.16, 17 Full characterization has previously been reported,18 particularly the degree of branching (that is, 0.57 according to Frey’s calculation method).19 The purpose of this study was to use the hyperbranched poly(siloxysilane) as a linker between PNIPAM and the glass surface by simply drop casting of it onto the surface of glass slides.
The possibility of adhesion onto different surfaces by simple drop casting is one of the main advantages of this system compared with previous methodologies. The resulting coated surface can be applied to thermoresponsive applications, such as cell culture. Indeed, some cells cannot be grown on thermoresponsive PSt dishes, currently one of the most common systems for growing cell sheets.
In this study, we report the synthesis of inorganic–organic hyperbranched poly(siloxysilane) grafted with poly(N-isopropylacrylamide), and its casting onto glass slides. The coated surface has been successfully used for cell culture of mouse 3T3 fibroblast cells in sheets followed by detachment of the sheet by cooling to room temperature.
Experimental procedures
Measurements
The nuclear magnetic resonance (NMR) (1H, 400 MHz) spectra were recorded for samples dissolved in chloroform-d using a JEOL JNM-ECS 400 NMR spectrometer (JEOL Ltd., Tokyo, Japan). The molecular weight of the prepared HBPS was determined by gel permeation chromatography (GPC) relative to polystyrene standards using a Shodex GPC-101 system equipped with a refractive index detector and an LF-804 column (Showa Denko, Tokyo, Japan), and tetrahydrofuran (THF) was used as the eluent. The molecular weight of PNIPAM was determined by GPC relative to polystyrene standards using a Viscotek TDA 302 system (Malvern Instruments Ltd, Worcestershire, UK) with a refractive index detector, an LF-804 column and N,N-dimethylformamide containing 50 mM of lithium bromide as the eluent. The elemental composition was determined using a CHNS/O 2400-II analyzer (PerkinElmer, Waltham, MA, USA). X-ray photoelectron spectroscopy was conducted using a JSP-9010MC (JEOL) spectrometer with an aluminum target. Tapping mode atomic force microscopy measurements were performed on a Seiko model SPA-400 (Seiko Instruments Inc., Chiba, Japan). The contact angles were measured using a Kyowa DM-501YH (Kyowa Interface Science Co., Ltd., Saitama, Japan). The reported contact angle values are an average of 5 measurements from 3 different glass slides using 2 μl of pure water, and these values were recorded after 21 s. Before measuring the contact angle at 50 °C, the glass slides were preconditioned at 50 °C for 1 h.
Materials
A 0.1 M solution of the platinum 1,3-divinyl-1,1,3,3-tetramethyldisiloxane complex (Pt[dvs]) in xylene and a 0.5 M 9-borabicyclo[3.3.1]-nonane solution in THF were purchased from Sigma-Aldrich (Tokyo, Japan). NIPAM was obtained from TCI (Tokyo, Japan) and purified by recrystallization from hexane twice. A hydrogen peroxide solution (35% in water), 4-dimethylaminopyridine and N,N’-dicyclohexyl carbodiimide were purchased from TCI and used as received. 2,2′-Azobis(2-methylpropionitrile) (AIBN) was purchased from Wako (Osaka, Japan) and recrystallized from methanol.
We have previously reported the synthesis of vinyl-terminated hyperbranched poly(siloxysilane) from 1,5-divinyl-1,1,3,5,5-pentamethyltrisiloxane (AB2 monomer).20 The synthesis of S-1-dodecyl-S′-(α,α′-dimethyl-α′′-acetic acid) trithiocarbonate has also been previously reported.21
Micro glass slides (size: 26 × 76 mm, 0.8–11.0 mm in thickness, frosted 15 × 26 mm) were obtained from Matsunami Glass (Osaka, Japan) and treated with a piranha solution (70% H2SO4, 30% H2O2) before surface modification to remove impurities from the surface and maximize the number of hydroxyl groups present at the surface.
Synthesis of HBPS-OH P1
The synthesis of hydroxyl-terminated hyperbranched poly(siloxysilane) (HBPS-OH) P1 was performed according to a previously reported procedure with minor modifications.18 P1 was obtained as a colorless viscous liquid (Mn: 9 900 g mol−1, PDI: 2.0). 1H NMR: δ −0.10 (br, Si(CH3)CHSi), 0.07 (br, OSi(CH3)2), 0.11 (br, SiCH3O), 0.42 (br, SiC2H4), 0.93 (br, Si(CH3)CHSi), 1.03 (br, SiCH2CH2O) and 3.77 (br, SiCH2CH2O) p.p.m.
Synthesis of CTA-terminated hyperbranched poly(siloxysilane) P2
The chain transfer agent (CTA)-terminated hyperbranched poly(siloxysilane) (HBPS-CTA) P2 was synthesized to a previously reported procedure with minor modifications.15 P2 was obtained as an orange, viscous liquid (Mn: 5 600 g mol−1, PDI: 1.7). 1H NMR: δ −0.10 (br, Si(CH3)CHSi), 0.07 (br, OSi(CH3)2), 0.11 (br, SiCH3O), 0.44 (br, SiCH2CH2O), 0.88 (t, SC10H20CH3, 2.6 Hz), 0.98 (br, Si(CH3)CHSi), 1.02 (br, SiCH2CH2O), 1.25 (br, SC2H4C9H18CH3), 1.58 (s, SCH2CH2), 1.67 (br, C(CH3)2), 3.26 (t, SCH2, 7.6 Hz) and 4.21 (br, SiCH2CH2O) p.p.m.
Synthesis of hyperbranched poly(siloxysilane) grafted poly(N-isopropylacrylamide) P3
HBPS-CTA P2 (52.5 mg), AIBN (1.1 mg) and NIPAM (1.85 g, 16.38 mmol, 190 eq) were dissolved in 13 ml of THF. The tube was sealed under vacuum by using a freeze-vacuum-thaw method. Then, the tube was placed in an oil bath at 65 °C, and the mixture was vigorously stirred for 24 h. The mixture was cooled to 0 °C and immediately diluted with THF. Removal of the solvent afforded the crude product that was precipitated three times from acetone into diethyl ether. The precipitate was collected and dried in vacuum to yield HBPS-g-PNIPAM P3 as a white powder (PNIPAM content: 85%). 1H NMR: δ 0.07 (br, OSi(CH3)2), 0.11 (br, SiCH3O), 0.44 (br, SiCH2CH2O), 0.88 (t, SC10H20CH3, 2.6 Hz), 1.15 (b, NHCH(CH3)2), 1.25 (br, SC2H4C9H18CH3), 1.72 (br, CH2CHCO), 2.24 (br, CH2CHCO) and 3.99 (br, NHCH(CH3)2) p.p.m.
Surface modification of glass plates
A commercial glass slide was pretreated with 30 ml of a piranha solution (70% H2SO4, 30% H2O2) at 80 °C for 30 min, and this was followed by thorough washing with water and ethanol. A solution consisting of HBPS-g-PNIPAM P3 in THF was drop casted onto the glass slide and dried in air for 24 h, and this was followed by heat treatment at 100 °C for 24 h. Any unattached and poorly physisorbed polymer was washed off by submerging the plate in water for 24 h and the plate was then dried in air.
Cell culture experiments
Mouse 3T3 fibroblast cells were seeded on the polymer-coated dish according to the following procedure. Then, 2 ml of medium (Dulbecco’s modified Eagle’s medium with 10% fetal calf serum was placed into a polymer-coated dish, and medium containing 1 × 105 mouse 3T3 fibroblast cells was added. Cells were cultivated in a CO2 incubator (37 °C, 5% CO2). After 1 day, the surface of the culture dish was observed with an optical microscope while the temperature was maintained at 37 °C. Then, the sample was placed in an incubator (20 °C, 5% CO2) for 15 min, and the surface was observed again. Another set of samples, which were prepared through the same procedure, was placed in an incubator for 4 days, and the surface was observed in the same manner after cooling.
Results and discussion
Synthesis of hyperbranched poly(siloxysilane) grafted poly(N-isopropylacrylamide)
PNIPAM-grafted hyperbranched poly(siloxysilane) HBPS-g-PNIPAM was obtained by the reversible addition–fragmentation chain transfer (RAFT) polymerization from HBPS-CTA, as shown in Scheme 1.
The vinyl-terminated hyperbranched polymer was obtained by hydrosilylation of the AB2 monomer in the presence of Pt(dvs) that was used as a catalyst. Hydroboration of the double bonds followed by treatment with hydrogen peroxide led to the formation of HBPS-OH P1. Steglich esterification of the hydroxyl group of the hyperbranched polymer with trithiocarbonate CTA yielded HBPS-CTA P2. Elemental analysis of HBPS-CTA P2 indicated that only 20% of the end groups bore the CTA agent. However, owing to the possibility of crosslinking between end groups, this result does not indicate that the remaining 80% of the end groups possess hydroxyl functions. Indeed, the termini of hyperbranched poly(siloxysilane) tend to react intramolecularly, thus leading to fewer end groups than expected.22 In addition, after introduction of CTA onto the end groups of the hyperbranched polymer, the overall molecular weight decreased because of crosslinking, which occurred during end groups modification, and the resulting crosslinked polymer was washed out during the purification process.
HBPS-g-PNIPAM P3 was synthesized by RAFT polymerization of NIPAM from HBPS-CTA P2 in the presence of AIBN as an initiator in THF at 65 °C for 24 h. The 1H-NMR analysis of HBPS-g-PNIPAM P3 (Figure 1) indicated the presence of characteristic peaks from PNIPAM at 1.15 (br, NHCH(CH3)2), 1.72 (br, CH2CHNH), 2.24 (br, CH2CHNH) and 3.99 (br, NHCH(CH3)2) p.p.m.; HBPS at 0.44 (br, SiC2H4Si) and 0.07 (br, OSi(CH3)2C) p.p.m.; the alkyl chain of the CTA at 1.25 p.p.m.; and the methyl group of the alkyl chain at 0.88 p.p.m. Elemental analysis of HBPS-g-PNIPAM P3 permits the calculation of the PNIPAM content that was 88%.
The PNIPAM segment was cleaved from the hyperbranched polymer by hydrolysis of the ester linkage using an aqueous NaOH solution, and this was followed by isolated by precipitation in hexane. GPC analysis of the cleaved PNIPAM yielded a molecular weight of 10 400 g mol−1 with a narrow polydispersity of 1.2, thus indicating the good livingness of the RAFT polymerization in our system.
Glass surface modification
HBPS-g-PNIPAM P3 solutions in THF were casted onto the surface and dried in air for 24 h. Then, the glass slides were heated to 100 °C for 24 h to maximize the interaction and promote crosslinking between the hydroxyl groups of the surface and the siloxane part of the hyperbranched polymer as well as crosslinking of the hyperbranched polymer. The chemical bonding interface layer is formed by condensation of the hydroxyl group on the surface with siloxane groups.23 To remove unattached and poorly attached polymers, the glass plates were submerged in ultrapure water for 24 h and then dried in an oven at 50 °C overnight.
The contact angles of the uncoated and P3-coated glass slides were measured at 20 and 50 °C, and the results are summarized in Table 1. After piranha treatment, the surface of the glass plate was hydrophilic with a contact angle of ∼22.5°. However, after P3 treatment, the contact angle was ∼58°, and this is slightly less than that reported in a previous study of a PNIPAM-coated surface under the LCST (that is, ∼79° at 15 °C)11 for the use of linear poly(silsesquioxanes) linker-grafted PNIPAM. After the temperature increased to 50 °C, the contact angle of the P3-coated glass slide was 67°, higher than the contact angle measured at 20 °C. The contact angle is expected to increase when the temperature is higher than the LCST of PNIPAM (~32 °C), owing to a shift in the PNIPAM chain from hydrophilic to hydrophobic, resulting from a coil-to-globule transition.6
The X-ray photoelectron spectroscopy results for the uncoated and P3-coated glass slides are shown in Figure 2. The survey of the uncoated glass slide indicated the presence of only three elements (that is, silicon (2p3/2, 103 eV), carbon (1 s, 284 eV) and oxygen (1 s, 533 eV)), a result that is consistent with the composition of the glass slide. Coating of P3 onto the surface of the glass slide resulted in the observation of a characteristic peak corresponding to nitrogen (1 s, 399.5 eV), owing to the PNIPAM segment. The ratio of the nitrogen peak to the silicon peak for the P3-coated glass slide was ∼8 times higher than that of the uncoated slide glass.
The atomic force microscopy images of the uncoated and P3-coated surface are shown in Figure 3. The surface of the uncoated glass slide has a very flat surface. However, several dust particles were observed during the measurement. The surface of the P3-coated glass slide indicated the presence of small particles as the size of P3 increased from 15 to 45 nm. The size difference may be explained by the size of the aggregates (that is, from single-molecule modification to aggregate of several HBPS on the surface).
These experimental observations suggest that the glass slide was successfully modified with PNIPAM.
Cell culture
Figures 4a–f show the proliferation behavior at 37 °C after 2 and 4 days of culture as well as the detachment behavior at 20 °C for 15 min after 4 days of culture on the uncoated (Figures 4a–c) and P3-coated (Figures 4d–f) glass slides. At 37 °C, the cells adhered to the uncoated slide glass, and proliferation was possible after 2 days. In addition, a cell sheet was formed after 4 days of culture. However, the cell sheet could not detach from the surface owing to the strong interaction between the hydrophobic slide glass surface and the proteins from the medium as well as the lack of a thermoresponsive surface. The adhesion of the P3-coated surface was achieved, and similar to the uncoated glass slide, the cell sheet was obtained after 4 days of culture. On the coated surface, in contrast to the uncoated surface, detachment of the cell sheet was possible after cooling at 20 °C for 15 min owing to the presence of PNIPAM segments on the surface of the P3-coated glass slide. Therefore, the P3-coated surface formed by drop casting is suitable for thermoresponsive cell culture.
Previous characterization results have suggested that P3 can successfully attach onto the surface of the glass slide. However, it is important to determine whether the coating layer can survive and maintain its efficiency after cell culture. To assess the reusability of our system, cells were seeded on the P3-coated glass slide for a second time. After 2 days of culture, the cells adhered and proliferated on the surface of the glass slide (image Figure 4g). A cell sheet was obtained after 4 days of culture, and was able to be detached by cooling to 20 °C for 15 min (images Figures 4h and i). The successful reusability of the P3-coated glass slide suggests that P3 is strongly attached to the surface by simple drop casting of a P3 solution in THF onto the slide.
Conclusion
Hyperbranched poly(siloxysilane) grafted poly(N-isopropylacrylamide) P3 was successfully synthesized by RAFT polymerization of NIPAM from the CTA termini of HBPS. The glass slides were PNIPAM modified by simply casting a solution of P3 in THF onto a commercial slide glass, as confirmed by contact angle, X-ray photoelectron spectroscopy and atomic force microscopy measurements.
Mouse 3T3 fibroblast cells were cultured on the PNIPAM-modified glass plate at 37 °C for 2 and 4 days. The cells proliferated and formed cell sheets that were successfully detached from the surfaces by cooling the glass slide to 20 °C for 15 min. Strong adhesion between P3 and the glass slide was suggested by the successful of the P3-coated glass slide for cell culture.
In the future, we plan to test our PNIPAM-coated slide glass with different cells that require glass surfaces for proliferation. In addition, to ensure wider surface coating possibilities, HBPS-g-PNIPAM will be tested for coating other useful surfaces for cell culture.

Synthesis of hyperbranched poly(siloxysilane) grafted poly(N-isopropylacrylamide).
References
Shimizu, T., Yamato, M., Isoi, Y., Akutsu, T., Setomaru, T., Abe, K., Kikuchi, A., Umezu, M. & Okano, T. Fabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfaces. Circ. Res. 90, 40e–48e (2002).
Nishida, K., Yamato, M., Hayashida, Y., Watanabe, K., Yamamoto, K., Adachi, E., Nagai, S., Kikuchi, A., Maeda, N., Watanabe, H., Okano, T. & Tano, Y. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N. Engl. J. Med. 351, 1187–1196 (2004).
Yamada, N., Okano, T., Saki, H., Karikusa, F., Sawasaki, Y. & Sakurai, Y. Thermo- responsive polymeric surfaces; control of attachment and detachment of cultured cells. Die Makromol. Chem. Rapid Commun. 11, 571–576 (1990).
Okano, T., Yamada, N., Okuhara, M., Sakai, H. & Sakurai, Y. Mechanism of cell detachment from temperature-modulated, hydrophilic-hydrophobic polymer surfaces. Biomater. Silver Jubil. Compend. 16, 297–303 (1995).
Ebara, M., Yamato, M., Aoyagi, T., Kikuchi, A., Sakai, K. & Okano, T. Immobilization of cell-adhesive peptides to temperature-responsive surfaces facilitates both serum-free cell adhesion and noninvasive cell harvest. Tissue Eng. 10, 1125–1135 (2004).
Heskins, M. & Guillet, J. E. Solution Properties of Poly(N-isopropylacrylamide) J. Macromol. Sci. A 2, 1441–1455 (1968).
Takahashi, H., Nakayama, M., Yamato, M. & Okano, T. Controlled chain length and graft density of thermoresponsive polymer brushes for optimizing cell sheet harvest. Biomacromolecules 11, 1991–1999 (2010).
Takahashi, H., Nakayama, M., Itoga, K., Yamato, M. & Okano, T. Micropatterned thermoresponsive polymer brush surfaces for fabricating cell sheets with well-controlled orientational structures. Biomacromolecules 12, 1414–1418 (2011).
Tamura, A., Nishi, M., Kobayashi, J., Nagase, K., Yajima, H., Yamato, M. & Okano, T. Simultaneous enhancement of cell proliferation and thermally induced harvest efficiency based on temperature-responsive cationic copolymer-grafted microcarriers. Biomacromolecules 13, 1765–1773 (2012).
Sudo, Y., Sakai, H., Nabae, Y., Hayakawa, T. & Kakimoto, M. Preparation of hyperbranched polystyrene-g-poly(N-isopropylacrylamide) copolymers and its application to novel thermo-responsive cell culture dishes. Polymer 70, 307–314 (2015).
Kessler, D. & Theato, P. Reactive surface coatings based on polysilsesquioxanes: defined adjustment of surface wettability. Langmuir 25, 14200–14206 (2009).
Kessler, D., Nilles, K. & Theato, P. Modular approach towards multi-functional surfaces with adjustable and dual-responsive wettability using a hybrid polymer toolbox. J. Mater. Chem. 19, 8184–8189 (2009).
Wagner, N., Kessler, D. & Theato, P. Reactive coatings in glass capillaries: preparation of temperature- and light-responsive surfaces and accurate determination of wettability Switching. Macromol. Chem. Phys. 217, 92–100 (2016).
Yamada, Y., Hirai, T., Kikuchi, R., Hayakawa, T. & Kakimoto, M. Adsorption of hyperbranched polysiloxysilane modified with triethoxy group onto the silicon wafer. High Perform. Polym. 19, 700–710 (2007).
Park, B. R., Nabae, Y., Surapati, M., Hayakawa, T. & Kakimoto, M. Poly(N-isopropylacrylamide)-modified silica beads with hyperbranched polysiloxysilane for three-dimensional cell cultivation. Polym. J. 45, 210–215 (2012).
Nogami, K., Kakimoto, M., Hayakawa, T., Yokomachi, K., Seino, M. & Sakamoto, K. Modification of anodized aluminum film by hyperbranched poly(siloxysilane)s in conductive polymer aluminum solid electrolytic capacitor. Chem. Lett. 35, 134–135 (2006).
Nogami, K., Sakamoto, K., Hayakawa, T. & Kakimoto, M. The effects of hyperbranched poly(siloxysilane)s on conductive polymer aluminum solid electrolytic capacitors. J. Power Sources 166, 584–589 (2007).
Yokomachi, K., Seino, M., Grunzinger, S. J., Hayakawa, T. & Kakimoto, M. Synthesis and degree of branching of epoxy-terminated hyperbranched polysiloxysilane. Polym. J. 40, 198–204 (2008).
Frey, H., Hölter, D. & Burgath, A. Degree of branching in hyperbranched polymers. Acta Polym. 48, 30–35 (1997).
Seino, M., Yokomachi, K., Hayakawa, T., Kikuchi, R., Kakimoto, M. & Horiuchi, S. Preparation of poly(N-isopropylacrylamide) grafted silica bead using hyperbranched polysiloxysilane as polymer brush and application to temperature-responsive HPLC. Polymer 47, 1946–1952 (2006).
Skey, J. & O’Reilly, R. K. Facile one spot synthesis of a range of reversible addition-fragmentation chain transfer (RAFT) agents. Chem. Commun. 35, 4183–4185 (2008).
Percec, V., Chu, P. & Kawasumi, M. Toward ‘willowlike’ thermotropic dendrimers. Macromolecules 27, 4441–4453 (1994).
Pulker, H. K., Perry, A. J. & Berger, R. Adhesion. Surf. Technol. 14, 25–39 (1981).
Acknowledgements
RG is supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Gillet, R., Sakai, H., Nabae, Y. et al. Synthesis of hyperbranched-linear poly(N-isopropylacrylamide) polymers with a poly(siloxysilane) hyperbranched macroinitiator, and their application to cell culture on glass substrates. Polym J 48, 1007–1012 (2016). https://doi.org/10.1038/pj.2016.64
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2016.64