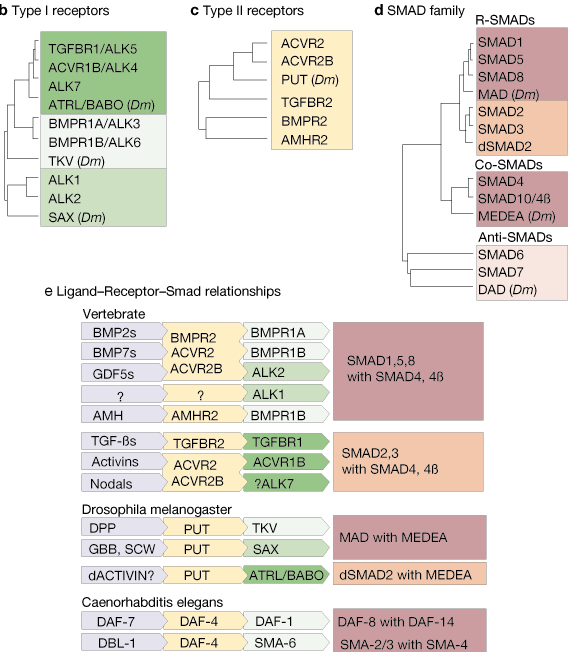
The TGF-b system is highly conserved throughout the animal kingdom. As described in the accompanying review1, the basic pathway provides a simple route for signals to pass from the extracellular environment to the nucleus, involving only four types of molecule:
| � TGF-b-family growth factors (blue) |
| � Type I receptors (green) |
| � Type II receptors (yellow) |
| � SMAD transcription factors (red) |
It includes orthologues from several model organisms — mammals (human unless otherwise indicated), African clawed frog (Xenopus laevis), zebrafish (Danio rerio), fruitfly (Drosophila melanogaster) and nematode worm (Caenorhabditis elegans) — and provides links to appropriate sequence databases for each molecule. The dendrograms represent the level of structural similarity between the members of the TGF-b; family (bioactive domains only), receptor families (kinase domains only) and SMADs.
a | Extracellular TGF-b-family ligands transmit their signals to the cell's interior by binding to c | type II receptors, which form heterodimers with b | type I receptors. This activates the receptor's serine/threonine kinase activity to phosphorylate and activate d | SMAD transcription factors. Phosphorylation of receptor-activated SMADs (R-SMADs) by the receptor dimer allows the R-SMADs to form heterodimers with partner SMADs (co-SMADs) and translocate to the nucleus where, in collaboration with a host of other factors, they activate or inhibit the transcription of target genes (see accompanying review1 for more information).
Details of some of the interactions shown in part e have yet to be fully established. Although BMP2-, BMP7- and GDF2-family members interact with the type II receptors indicated which, in turn, interact with the type I receptors indicated, not all of the possible ligand-receptor combinations have been shown to occur. For example, BMP4 is the only member of the BMP2 subfamily whose interactions with different type II receptors have been tested. Similarly, although ALK7 has been shown to activate SMAD2 (using a constitutively active ALK7 mutant), the ligand and corresponding type II receptor for ALK7 remain unknown. However, it is more likely that they interact with the activin receptors ACVR2 and ACVR2B than with TGFBR2 because the latter is very specific for TGF-b, whereas ACVR2 and ACVR2B are more promiscuous.
Abbreviations: ACVR, activin receptor; ADMP, anti-dorsalizing morphogenetic protein; ALK, activin receptor-like kinase; AMH, anti-M�llerian hormone; AMHR, AMH receptor; ATRL, activin receptor-like; BABO, baboon; BMP, bone morphogenetic protein; BMPR, BMP receptor; DPP, decapentaplegic; EBAF, endothelial bleeding associated factor; GBB, glass-bottom boat; GDF, growth and differentiation factor; MIS, M�llerian inhibiting substance; Ndr, nodal-related factor; OP, osteogenic protein; PUT, punt; SAX, saxophone; SCW, screw; TGF, transforming growth factor; TGFBR, TGF-b receptor; TKV, thickveins; Vgr, Vg-related; Xnr, Xenopus nodal-related factor; zDVR: zebrafish decapentaplegic and Vg1-related.
Clicking on any protein name in the diagram takes you directly to the relevant entry in one of the following databases:
1 Massague, J. How cells read TGF-ß signals. Nature Rev. Mol. Cell. Biol. 1, 169-178 (2000). [Full text]

![]()
![]() Linking by Cath Brooksbank and Nick Allin. Artwork by Nicola Barker.
Linking by Cath Brooksbank and Nick Allin. Artwork by Nicola Barker.