Abstract
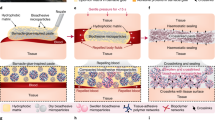
Bleeding is largely unavoidable following syringe needle puncture of biological tissues and, while inconvenient, this typically causes little or no harm in healthy individuals. However, there are certain circumstances where syringe injections can have more significant side effects, such as uncontrolled bleeding in those with haemophilia, coagulopathy, or the transmission of infectious diseases through contaminated blood. Herein, we present a haemostatic hypodermic needle able to prevent bleeding following tissue puncture. The surface of the needle is coated with partially crosslinked catechol-functionalized chitosan that undergoes a solid-to-gel phase transition in situ to seal punctured tissues. Testing the capabilities of these haemostatic needles, we report complete prevention of blood loss following intravenous and intramuscular injections in animal models, and 100% survival in haemophiliac mice following syringe puncture of the jugular vein. Such self-sealing haemostatic needles and adhesive coatings may therefore help to prevent complications associated with bleeding in more clinical settings.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Pereira, J. & Phan, T. Management of bleeding in patients with advanced cancer. Oncologist 9, 561–570 (2004).
Prandoni, P. et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 100, 3484–3488 (2002).
Domm, J. A., Hudson, M. G. & Janco, R. L. Complications of central venous access devices in paediatric haemophilia patients. Haemophilia 9, 50–56 (2003).
Doerfler, M. E., Kaufman, B. & Goldenberg, A. S. Central venous catheter placement in patients with disorders of haemostasis. Chest 110, 185–188 (1996).
Srivastava, A. et al. Guidelines for the management of haemophilia. Haemophilia 19, 1–47 (2013).
Chee, Y. L., Crawford, J. C., Watson, H. G. & Greaves, M. Guidelines on the assessment of bleeding risk prior to surgery or invasive procedures. Br. J. Haematol. 140, 496–504 (2008).
De Boulle, K. & Heydenrych, I. Patient factors influencing dermal filler complications: prevention, assessment, and treatment. Clin. Cosmet. Invest. Dermatol. 8, 205–214 (2015).
Batorova, A. & Martinowitz, U. Intermittent injections vs. continuous infusion of Factor VIII in haemophilia patients undergoing major surgery. Br. J. Haematol. 110, 715–720 (2000).
World Federation of Hemophilia Report on the Annual Global Survey 2014 (World Federation of Hemophilia, 2015).
Plug, I. et al. Mortality and causes of death in patients with haemophilia, 1992–2001 a prospective cohort study. J. Thromb. Haemost. 4, 510–516 (2006).
Prausnitz, M. R. Microneedles for transdermal drug delivery. Adv. Drug Deliv. Rev. 56, 581–587 (2004).
Sherman, A. et al. Suppression of inhibitor formation against FVIII in a murine model of haemophilia A by oral delivery of antigens bioencapsulated in plant cells. Blood 124, 1659–1668 (2014).
Lisbeth Illum, L. Nasal drug delivery-possibilities, problems and solutions. J. Control. Release 87, 187–198 (2003).
Hong, S. et al. Air/Water interfacial formation of freestanding, stimuli-responsive, self-healing catecholamine Janus-faced microfilms. Adv. Mater. 26, 7581–7587 (2014).
Ryu, J. H., Jo, S., Koh, M. & Lee, H. Bio-inspired, water-soluble to insoluble self-conversion for flexible, biocompatible, transparent, catecholamine polysaccharide thin films. Adv. Funct. Mater. 24, 7709–7716 (2014).
Kastrup, C. J. et al. Painting blood vessels and atherosclerotic plaques with an adhesive drug depot. Proc. Natl Acad. Sci. USA 109, 21444–21449 (2012).
Ong, S.-Y., Wu, J., Moochhala, S. M., Tan, M.-H. & Lu, J. Development of a chitosan-based wound dressing with improved haemostatic and antimicrobial properties. Biomaterials 29, 4323–4332 (2008).
Whang, H. S., Kirsch, W., Zhu, Y. H., Yang, C. Z. & Hudson, S. M. Haemostatic agents derived from chitin and chitosan. J. Macromol. Sci. Polym. Rev. 45, 309–323 (2005).
Kim, K., Kim, K., Ryu, J. H. & Lee, H. Chitosan-catechol: a polymer with long-lasting mucoadhesive properties. Biomaterials 52, 161–170 (2015).
Ryu, J. H. et al. Catechol-functionalized chitosan/pluronic hydrogels for issue adhesives and haemostatic materials. Biomacromolecules 12, 2633–2659 (2011).
Mizoguchi, Y. et al. Changes in blood parameters in New Zealand White rabbits during pregnancy. Lab. Anim. 44, 33–39 (2010).
Russell, K. E. et al. Reduced bleeding events with subcutaneous administration of recombinant human factor IX in immune-tolerant haemophilia B dogs. Blood 102, 4393–4398 (2003).
Hauck, T. S., Anderson, R. E., Fischer, H. C., Newbigging, S. & Chan, W. C. W. In vivo quantum-dot toxicity assessment. Small 6, 138–144 (2010).
Zhang, X.-D. et al. Toxicologic effects of gold nanoparticles in vivo by different administration routes. Int. J. Nanomedicine 5, 771–781 (2010).
Zhang, Y., Thomas, Y., Kim, E. & Payne, G. F. pH- and voltage-responsive chitosan hydrogel through covalent cross-linking with catechol. J. Phys. Chem. B 116, 1579–1585 (2012).
Wang, S. X. et al. A crosslinked cofactor in lysyl oxidase: redox function for amino acid side chains. Science 273, 1078–1084 (1996).
Xia, J., Xu, Y., Lin, J. & Hu, B. UV-induced polymerization of urushiol without photoinitiator. Prog. Org. Coat. 61, 7–10 (2008).
Anh, N. V. & Williams, R. M. Bis-semiquinone (bi-radical) formation by photoinduced proton coupled electron transfer in covalently linked catechol–quinone systems: Aviram’s hemiquinones revisited. Photochem. Photobiol. Sci. 11, 957–961 (2012).
Tindale, C. R. Reactions of biogenic amines with quinones. Aust. J. Chem. 37, 611–617 (1984).
Xua, X., Wanga, L., Guoa, S., Lei, L. & Tang, T. Surface chemical study on the covalent attachment of hydroxypropyltrimethyl ammonium chloride chitosan to titanium surfaces. Appl. Surf. Sci. 257, 10520–10528 (2011).
Amaral, I. F., Granja, P. L. & Barbosa, M. A. Chemical modification of chitosan by phosphorylation: an XPS, FT-IR and SEM study. J. Biomater. Sci. Polym. Edn. 16, 1575–1593 (2005).
Lee, H., Scherer, N. F. & Messersmith, P. B. Single-molecule mechanics of mussel adhesion. Proc. Natl Acad. Sci. USA 103, 12999–13003 (2006).
Ahn, B. K., Lee, D. W., Israelachvili, J. N. & Waite, J. H. Surface-initiated self-healing of polymers in aqueous media. Nat. Mater. 13, 867–872 (2014).
Miller, D. R. et al. Mussel coating protein-derived complex coacervates mitigate frictional surface damage. ACS Biomater. Sci. Eng. 1, 1121–1128 (2015).
Bardelmeijer, H. A. et al. Cannulation of the jugular vein in mice: a method for serial withdrawal of blood samples. Lab. Anim. 37, 181–187 (2003).
Parasuraman, S., Raveendran, R. & Kesavan, R. Blood sample collection in small laboratory animals. J. Pharmacol. Pharmacother. 1, 87–93 (2010).
Bollard, C. M., Teague, L. R., Berry, E. W. & Ockelford, P. A. The use of central venous catheters (portacaths) in children with haemophilia. Haemophilia 6, 66–70 (2000).
Miller, K. et al. Implantable venous access devices in children with hemophilia: a report of low infection rates. J. Pediatr. 132, 934–938 (1998).
Hevener, A. L., Bergman, R. N. & Donovan, C. M. Novel glucosensor for hypoglycemic detection localized to the portal vein. Diabetes 46, 1521–1525 (1997).
Salgado, O. J., Urdaneta, B., Colmenares, B., García, R. & Flores, C. Right versus left internal jugular vein catheterization for hemodialysis: complications and impact on ipsilateral access creation. Artif. Organs 28, 728–733 (2004).
Acknowledgements
This work was financially supported from National Research Foundation of South Korea (NRF) from the Ministry of Future Creative Science: Mid-Career Scientist Grant (2014002855) and End-Run Program funded by KAIST. In addition, this research was also supported from the Korea Health Technology R&D Project through the Health Industry Development Institute (KHIDI) and National R&D Program for Cancer Control by the Ministry of Health & Welfare, South Korea (HI14C2755; S.-W.K. and 1631060; H.L.).
Author information
Authors and Affiliations
Contributions
M.S. conceived and designed the experiments. M.S., S.-G.P. and B.-C.O. performed the experiments. S.J. prepared CHI-C. M.S. and B.-C.O. performed the preparation and the characterization of the haemostatic needles. M.S., S.-G.P. and K.K. performed in vivo experiments. M.S., S.-G.P., K.-S.K., S.-W.K. and H.L. interpreted in vivo results. M.S., K.K. and S.-H.H. performed HCV-related experiments. S.-H.H and E.-C.S. discussed and interpreted the viral infection results. M.S. and H.L. wrote the paper. All authors discussed the results. M.S. and H.L. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1853 kb)
Supplementary Information
Supplementary movie 1 (MP4 1979 kb)
Supplementary Information
Supplementary movie 2 (MP4 1043 kb)
Supplementary Information
Supplementary movie 3 (MP4 4056 kb)
Supplementary Information
Supplementary movie 4 (MP4 6046 kb)
Rights and permissions
About this article
Cite this article
Shin, M., Park, SG., Oh, BC. et al. Complete prevention of blood loss with self-sealing haemostatic needles. Nature Mater 16, 147–152 (2017). https://doi.org/10.1038/nmat4758
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4758
This article is cited by
-
Catechol- and thiol-containing binder that aggregates granular xenografts in reconstructed bone defects by mimicking mussel wet adhesion
Biotechnology and Bioprocess Engineering (2024)
-
Coupling of Adhesion and Anti-Freezing Properties in Hydrogel Electrolytes for Low-Temperature Aqueous-Based Hybrid Capacitors
Nano-Micro Letters (2024)
-
Hierarchical Self-Assembly of Injectable Alginate Supramolecular Nanofibril Hydrogels for Hemostasis In Vivo
Advanced Fiber Materials (2024)
-
Synthesis and characterization of polyacrylamide-based biomimetic underwater adhesives
Monatshefte für Chemie - Chemical Monthly (2023)
-
Development of cellulosic-based hemostatic dressing with antibacterial activity
Fashion and Textiles (2022)