At 9:25 p.m. on Wednesday 15 October 2008, Jill Rafael-Fortney sat down at her home-office computer and wrote an e-mail to Michael Ostrowski, the chair of her department at Ohio State University in Columbus.
"Mike, I didn't get either of my grants. I just found out about the second one a few minutes ago. My career in research seems to be over. It is all I ever planned to do from the age of six, so I don't really have another well thought-out plan. Can we talk tomorrow?"
Rafael-Fortney had tried, and failed, to renew the R01 grant from the National Institutes of Health (NIH) that supported her work on mouse models of muscular dystrophy. She had tried, and failed, to get a new R01 grant to study a genetic abnormality that might be widespread in human heart failure. At nearly 39, she had run out of track.
Four months earlier, at 4:00 a.m. on 10 June, Darcy Kelley had opened her laptop and logged onto the NIH's grant-review website to find out whether her own R01 application had made it through. For Kelley, a 59-year-old professor at Columbia University in New York, it was her third and final attempt to renew the major grant that supported her studies of the brain circuitry that produces and decodes sounds. She knew the results should be posted any day — and she could not sleep.
When Kelley saw that her score was 135, her heart leapt: this was outstanding on a scale in which 100 is the highest and 500 the lowest. Then as her eyes travelled further, it sank. She had heard that the National Institute of Neurological Disorders and Stroke (NINDS) was only funding proposals that scored in the 10th percentile or higher. Hers was in the 10.6th.
 Click for larger image.
Click for larger image.That crushing moment of disappointment is something that countless NIH-funded scientists have shared. Between 1998 and 2003 the US Congress doubled the agency's budget to US$27.1 billion and research institutions went on a hiring boom, recruiting faculty members, postdocs and graduate students. As those scientists started and expanded labs of their own, they applied to the NIH for support. Many hoped to secure one of the three-to-five-year R01 grants that form the mainstay of biomedical research funding in the country. But just as the number of grant applications rocketed, the NIH budget flattened (see graphs). In 2000, scientists such as Rafael-Fortney and Kelley who were applying to renew a previously funded R01 had a 53% chance of success on their first submission; in 2008, according to recent NIH figures, that success rate had fallen below 24%.
There are few hard data about the types of researchers who are being squeezed out — but Rafael-Fortney and Kelley do not seem unusual. Both women run what were, even in the good times, relatively small labs. They have solid publication records, including a paper each in the Proceedings of the National Academy of Sciences in the past three years1, 2. "There are literally hundreds if not thousands of people" in Rafael-Fortney's position, says Chip Souba, dean of the College of Medicine at Ohio State University. "Never in my 18–20 years of being funded by the NIH did the funding cut-off leave such a large percentage of applicants unfunded." Stuart Firestein, one of Kelley's colleagues in Columbia's department of biological sciences, adds: "You get a score like Darcy's and you find yourself in an unfundable position, and you have to say that there's something wrong with the system, not the individual."
“You have to say that there's something wrong with the system, not the individual.”
Stuart Firestein
But others say that the system is working as it should: there has to be a line, and someone has to fall below it. "Sometimes there is a flaw in the review but usually the other proposals were just plain better," says Kathy Hudson, director of the Genetics and Public Policy Center of Johns Hopkins University in Washington DC and a former assistant director of the NIH's National Human Genome Research Institute. "We like to bemoan the limited NIH budget, and all of us who feed at the NIH trough see endless benefits to biomedical research, especially our own. But these are taxpayer dollars in hard economic times; it is not an entitlement."
Greg Simon, president of FasterCures, a group based in Washington DC that campaigns for innovation in research, says that the biomedical enterprise has outstripped the ability of the NIH and other agencies to support it, and that researchers now have to turn to private foundations and other sources of funding if they want to survive. "There has been an assumption from the way people were trained and educated that the government is in charge of full employment for research scientists," he says. "Those days are over."
Some aspects of the current NIH system have clearly placed Kelley and Rafael-Fortney at a disadvantage. Kelley would almost certainly be funded today were it not for a 2007 mandate from former NIH director Elias Zerhouni that the agency fund more investigators who had never received an R01 before. In the same funding round in which Kelley was rejected by NINDS for falling below the 10th percentile, first-time applicants needed to score only in the top 25th percentile to get through. Kelley says that the push to fund young investigators is "absolutely fair". But she also says that her situation is a "prime example of unintended consequences". She directs a highly competitive graduate neuroscience programme, and has launched dozens of successful scientists from her lab. "By supporting me, you ensure the flow of talented young investigators. If you knock me out, fewer are trained," she says.
 Jill Rafael-Fortney obtained bridge funding from Ohio State University to sustain her lab.OHIO STATE UNIV. MEDICAL CENTER
Jill Rafael-Fortney obtained bridge funding from Ohio State University to sustain her lab.OHIO STATE UNIV. MEDICAL CENTERRafael-Fortney may have been penalized because she proposed projects that were deemed too scientifically adventurous by grant reviewers, who tend to favour studies that they know will produce the promised results. "Peer-review committees tend to be conservative and in bad times they become very conservative," Simon says. In this sense, Rafael-Fortney thinks that she is being out-competed by 'superstar' labs that boast 30 people at the bench and have the resources to collect convincing experimental data before they submit, rather than, as she did, needing the grant money to even start. Both women were also functioning on a single R01 grant in a time of diminishing NIH resources, something Kelley concedes was a mistake. "You're supposed to have two grants," she says. "It is common sense."
The stories of Kelley, with her long track record, and Rafael-Fortney, an investigator still trying to make her name, offer a glimpse into the personal consequences of research funding that dries up. Both now face an uncertain future — and yet both still struggle to work out exactly where they went wrong. "What I have learned from this experience is that during these times, not all good science gets funded," Rafael-Fortney says. "I don't feel like there's something that 'if only I had known' I could have done this or that." Kelley sums up her predicament more simply, saying she was "sandbagged by bad luck".
A knockout start
Late in 1996, Rafael-Fortney could be found bent over a microscope, analysing muscle tissue from a new line of transgenic mice. As a postdoctoral fellow in the lab of Kay Davies at the University of Oxford, UK, she was working on a new mouse model of muscular dystrophy, one that improved on the mdx mouse that was widely used at the time. The mdx model lacks dystrophin, the muscle protein that is missing in humans with Duchenne muscular dystrophy, but the mouse looks and acts relatively normal. A graduate student in the lab, Anne Deconinck, had knocked out a second protein called utrophin, and Rafael-Fortney found that the double knockouts had the same characteristics as patients with muscular dystrophy. "They had short stature, they were hunched up, they had difficulty breathing, they dragged their hind limbs." They also died within a few weeks.
Muscular dystrophy was what Rafael-Fortney had wanted to work on ever since, as a six-year-old, she had watched the Jerry Lewis muscular-dystrophy telethon at her home in Bayonne, New Jersey. "There were kids my age in wheelchairs," she recalls. "From that point I said: that's what I'm going do with my life. I want to find a way to help these kids and change their lives."
“Sometimes there is a flaw in the review but usually the other proposals were just plain better.”
Kathy Hudson
The mouse model, published in Cell3, offered a path to such help. It became a valuable model for many muscular-dystrophy labs working to develop gene therapies against the disease. But Rafael-Fortney did not intend to be among them. "I didn't want to start a lab doing the same thing that ten other labs were doing," she says. "I really wanted to think of how else we could approach muscular dystrophy and neuromuscular diseases in general." To that end, she decided to focus on an intriguing, microscopic feature that distinguished the neurons of her double-knockout mice from those of the mdx mice. At the junctions where the neurons connect with muscles, one of the membranes was abnormally flat. Rafael-Fortney thought that the peculiarity might underlie a lot of their muscular-dystrophy-like symptoms, and she wanted to launch a lab to investigate.
Late in 1999, she landed a tenure-track position in the Department of Molecular and Cellular Biochemistry at Ohio State University. She got a flying start with a $400,000, three-year Burroughs Wellcome Career Award, plus $500,000 worth of awards from the American Heart Association and the Muscular Dystrophy Association. By her 31st birthday a year later she had 26 publications under her belt, ample funding and a scientific mystery to solve that completely captivated her. She knew that she needed support from the NIH as well — "it was made clear from the moment you walk in the door for a faculty position that you would not get tenure until you get an R01," she says — but this didn't seem too much of an obstacle. Congress was serving up annual increases of 15% for the biomedical agency as part of the budget doubling. Everything seemed more than on track.
The Nobel dream
At the age of 11, Kelley read a book about Nobel prizewinners and began planning her experiments. At the age of 59 she was there in Stockholm, drinking champagne and waltzing in a gold-tiled ballroom — but as a guest of her friend, department chair and Nobel prizewinner Martin Chalfie, and keenly aware that despite 108 papers, her own research career was in jeopardy.
Kelley has been at Columbia since 1982. She works mainly with frogs of the genus Xenopus, some 500 of which she keeps in humid, plastic tanks across the hall from her lab. Xenopus is valued by researchers because of its underwater vocalizations, which involve only one muscle and are not complicated by the animal's need to breathe. Kelley has published work showing that sex hormones called androgens act both in auditory brain regions and in muscle fibres in the larynx to create the differing calls of males and females4, 5. She has also shown that an isolated larnyx6 and a larynx connected to a brain7 can 'sing' in a petri dish, allowing researchers to study the generation of sounds while dissecting the neural circuits involved.
 After nearly 30 years of NIH support, Darcy Kelley struggled to renew her major R01 grant.K. FRANK
After nearly 30 years of NIH support, Darcy Kelley struggled to renew her major R01 grant.K. FRANKIn 1988 and again in 1995, Kelley won two coveted Jacob Javits Neuroscience Investigator Awards from NINDS, in which 'highly productive' scientists are selected for seven years of R01 funding. Between 1983 and 1996, Kelley also operated on a second R01 from NINDS. And she has thrown as much energy into teaching as she has into research. Fourteen years ago, she co-founded Columbia's doctoral programme in neurobiology and behaviour, which is now one of the country's most sought-after graduate programmes in neuroscience. And in 2002, she was one of 20 scientists singled out for teaching excellence by the Howard Hughes Medical Institute and awarded $1 million over four years to do innovative things with undergraduate science education. That year, Kelley had also successfully renewed her Jacob Javitz award as a standard, five-year R01. Although her second R01 had lapsed and the NIH's budget was flattening, the Howard Hughes support made her feel secure, and she used it to support undergraduate research in the lab. "I can run my lab off of one R01. And I do a bunch of other stuff. I train students. I teach. One R01 makes sense," she says.
One R01 certainly seemed a good starting place to Rafael-Fortney in 2002 when, three years into her tenure-track job at Ohio, she got one. The National Institute of Arthritis and Musculoskeletal and Skin Diseases would pay her $910,000 over five years. The award was timely, beginning the very day after her funding from Burroughs Wellcome expired.
Because she was keen to carve out an original research path, Rafael-Fortney had proposed a relatively bold project for her first R01. The double-knockout mice had shown that an abnormal neuromuscular junction might be contributing to muscular dystrophy, and now she wanted to understand which proteins might be essential there. A large body of work pointed towards two proteins, called DLG and CASK, that have established roles in clustering channels and receptors at synapses in the central nervous system. Rafael-Fortney proposed to engineer lines of mice that would overexpress a mutated version of one of the proteins so that it no longer functioned correctly. If, as she expected, these mice had a pathology similar to muscular dystrophy, she would have started dissecting pathways with potential bearing on a broad spectrum of neuromuscular disorders.
“There are literally hundreds if not thousands of people in Rafael-Fortney's position.”
Chip Souba
As the results came in, though, they were disappointing. The mice didn't have muscular dystrophy. And Rafael-Fortney, who now ran a five-person lab, was starting to feel the financial pinch. By the end of 2004, her Muscular Dystrophy Association and American Heart Association grants had expired. Around two years before an R01's expiration, some investigators would begin preparing their applications for renewal, knowing that it can take many months to go through rounds of revision and resubmission. But Rafael-Fortney felt an early attempt at renewal would be "doomed" because she was still waiting for convincing experimental data. By late 2005, she was also in the late stages of her second pregnancy, and "realistically I can't function really well when I'm super pregnant", she says.
But in May 2006, on the heels of a brief maternity leave, Rafael-Fortney started working flat-out to submit her renewal. She knew that it would be tough: that year, the agency had sustained its first absolute budget cut in 36 years. "Things are looking bad," a former mentor and NIH grant reviewer warned her that summer. Rafael-Fortney was still set on bold projects though, and she was convinced that DLG and CASK were important. She proposed a project that was more technically ambitious than that in the first incarnation of the grant: knocking out DLG and CASK entirely in mouse skeletal muscle to see if their loss caused muscular dystrophy, as she still suspected it would. The thought was that removing the proteins would reveal their function more clearly than creating mutated versions. That November, one month after winning tenure, she submitted her renewal application.
Early rejection
Kelley's sole R01 came due for renewal as she was in the throes of a divorce from her husband of 33 years. It was October 2006 and her R01 was set to expire in April 2007. But after the split, she found it almost impossible to focus on the application. "My brain was blown," she says. So it was no surprise to her when, three months later, her application was 'triaged'. It had been assessed by three or more peer reviewers but then returned to her without being scored by the full 'study section' of reviewers to which it was assigned. (Between 40% and 60% of grants are routinely triaged before each study section meets.) That rejection put her at a significant disadvantage as she started re-writing the application. "When it is not scored, they don't give you the nitty gritty details of what they want you to do to improve it," says Kelley. In her application, she had proposed experiments in her frogs that would record the electrophysiological responses of single forebrain cells that both receive auditory input and direct the appropriate vocal response.
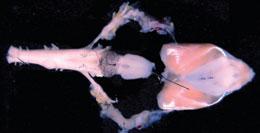 The isolated brain and larynx of the Xenopus frog can be used to study vocalization.E. ZORNIK/J. NEUROSCI.
The isolated brain and larynx of the Xenopus frog can be used to study vocalization.E. ZORNIK/J. NEUROSCI.Kelley knew that factors at the NIH were working against her. Institute directors were responding to Zerhouni's Road Map for Medical Research, with its emphasis on turning basic research into clinical application. And some of those that funded animal-communication work were starting to limit applications related to model organisms to those that were directly applicable to human disease. The result was a rise in the number of animal-communication applications assigned to NINDS, even as the institute's budget was stagnating. On top of that, Zerhouni had just issued his directive that a minimum number of awards must be given to investigators who had never won an R01 before.
Grant money is more than salaries. It is also lab animals and reagents — and being able to repair the −80° freezer when it breaks down. By the time Kelley's freezer failed on New Year's eve in 2007, it was almost eight months after her R01 had expired. She had already spent months paring right back. Earlier in the year, she had told her long-standing lab technician, Candace Barnard — a single mother of two who earned less than $40,000 a year — that her position would end. And as her postdocs and graduate students finished their projects and moved on, her molecular-biology workbenches fell quiet. Their experiments cost thousands of dollars to run, whereas those recording from live frogs cost hundreds. Even here, she watched every cent. Frogs cost $25 each, so she asked her remaining students to share them when possible. Kelley also did all the surgery on the frogs, fed them, changed the water in their tanks, washed glassware and made up solutions. When her freezer — crucial for preserving years of specimens — broke, she was glad that she had hoarded the $35,000 that Columbia's administrators had given her for equipment.
Making ends meet
Rafael-Fortney was also starting to cut back. In the same month that Kelley's R01 application was triaged, Rafael-Fortney learned that hers had been, too, and it was now a certainty that she would not win renewed funding before her current R01 expired in August 2007. She applied for — and received — $120,000 in bridge funding from the university that would keep her lab afloat while she was rewriting her application. "As people left, I let them leave, to try to make remaining funds last as long as possible," she says. Rafael-Fortney's freezer — already second-hand, and stuffed with mouse tissues — gave up in October 2008. By then she could not pay for the $4,000 repair, and she was forced to store her samples in the freezers of half-a-dozen colleagues.
“You're supposed to have two grants. It’s common sense.”
Throughout this time, Rafael-Fortney was writing and rewriting grant applications. The reviewers of her R01 renewal had said that the project was risky because the genetically engineered mice might not have a muscular-dystrophy phenotype. "We were asking for money to make the mouse, but they wanted it made already," she says. "It was a catch 22." But Rafael-Fortney had a second scientific string to her bow. Working with cardiac physiologist Paul Janssen from Ohio State University, she had shown that her double-knockout mice developed heart failure, which kills many patients with muscular dystrophy. One gene, called claudin 5, was expressed at strikingly low levels in the mice8 and, she found later, in 60% of samples from human hearts that had failed for all manner of reasons9. William Abraham, director of the Division of Cardiovascular Medicine at Ohio, recalls being "incredibly excited" when he first learned of Rafael-Fortney's work and urging her to patent the intellectual property (which she did). Her discovery, he said, "has the potential to be a game-changer".
Rafael-Fortney and Janssen decided to apply for a 'dual PI' award, a new grant mechanism that the NIH had been promoting in which an R01 award is led by two principal investigators. They wanted to tackle two questions with the $1.25 million they hoped to share over five years: could they create heart failure in a mouse by knocking out the claudin-5 gene; and could they rescue a mouse with heart failure by inserting the gene? In February 2007 they submitted their application.
The next few months were a rollercoaster of hope and rejection. The dual PI application was returned in June, unfunded, with a score of 186 on its first assessment. That was approaching the fundable range for these applications though, and the reviewers' comments were upbeat. Hugely encouraged, Rafael-Fortney and Janssen set to work responding to the comments and shaving costs before resubmitting. Then, in February 2008, the dual PI grant was returned again, this time with a score of 213, much lower than in the previous submission. Different experts had reviewed it this time, and they had different comments, suggesting, for example, that the clinical aims of the project be removed entirely. By this time, Rafael-Fortney's R01 renewal had been returned for the second time too, unscored. Now, she had only one 'strike' — one allowable attempt — left to win funding for each grant.
 Studies of the neuromuscular junction (top) could open up new angles for research on the disease.J. L SANFORD
Studies of the neuromuscular junction (top) could open up new angles for research on the disease.J. L SANFORDHer predicament became public in March 2008, when Rafael-Fortney was one of 12 young investigators to feature in a report entitled A Broken Pipeline?, compiled by leading US research institutions to plead the dire situation of young investigators. On the day of its release, Rafael-Fortney testified before the Senate Committee on Health, Education, Labor and Pensions at a hearing based on the report. "We're losing a generation of scientists," she told the senators. "They're people like me."
But both Rafael-Fortney and Kelley remained optimistic. Although Kelley's second renewal application had been rejected, the reviewers' comments had been positive, and she responded carefully. She submitted her third and final application on 5 March 2008. Her application was top-notch now, she felt, loaded with preliminary data and ripe for funding.
Rafael-Fortney, too, thought that her third application was convincing. With the help of another lab, she was able to generate a mouse line with CASK gene expression selectively knocked out in its skeletal muscle. Just days before the last application was due, she analysed the first of these mice. Their pathology showed that they did indeed have muscular dystrophy. She gave this top billing in her final application — she had completed a $30,000 piece of the proposed project, and had shown that the resulting mouse was a prime target for studying the disease. In effect, the application said "Look, we made the mice. We are really on to something," she says.
In the summer of 2008, Rafael-Fortney deferred a family vacation because there was no longer grant money to pay her summer salary. She submitted her final applications for both grants. Then she waited.
Desperate measures
On 10 June, the morning that Kelley learned that her third and final R01 application had failed, she started making phone calls. Merrill Mitler, the programme officer who was her main staff contact at NINDS replied by e-mail: the funding cut-off was "holding firm at the 10th percentile", he wrote, and her options there were limited. Kelley started chasing down Lana Shekim, the director of voice and speech programmes at the NIH's National Institute on Deafness and Other Communication Disorders. When she had submitted her renewal, Kelley had requested that the institute, which was funding down to the 21st percentile, consider funding her grant should it fail to make the cut at the hard-pressed neurology institute.
Shekim and Kelley finally connected by phone on 27 June. Kelley was in her car, returning to New York after giving a lecture at the Marine Biological Laboratory in Woods Hole, Massachusetts. She pulled over in a gravel parking lot, thinking it would be unwise to try to persuade an unknown bureaucrat to sustain her scientific future while behind the wheel.
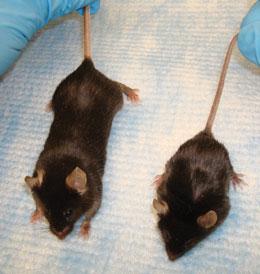 Mouse models of muscular dystrophy could open up new angles for research on the disease.J. L. SANFORD
Mouse models of muscular dystrophy could open up new angles for research on the disease.J. L. SANFORDShekim explained that Kelley's work was too far outside the institute's normal portfolio, which limited the animal work it funded to models of specific human disorders. "The science in this proposal does not directly address voice and speech disorders," Shekim later told Nature. Kelley disagreed — arguing that Xenopus can reveal conserved mechanisms by which nerve circuits generate vocal patterns. "I yelled at Lana. I said 'Look, I'm fighting for my scientific life here. I don't think you guys want to knock me out of science.'" Finally, a worn-out Kelley hung up and finished the long drive to New York.
A visitor to Kelley's lab last November would have found almost all her workbenches empty. One of her two remaining graduate students — whose $28,000 annual stipend the university had agreed to start paying — was preparing a list of needed supplies. A colleague of Kelley's was quietly paying for the supplies: the lab was effectively broke. Kelley knows now that she should have kept a second supporting grant in place. "The NIH funding programme takes people like me who run small labs, and have only a single grant, and penalizes them basically," she says. "Because they are not playing the game. They are not playing this multi-grant game." But others say that researchers such as Kelley need to anticipate how the funding environment will change and adjust their strategy accordingly. "If the NIH is turning towards a more clinically focused and translational-medicine emphasis, then it is researchers' responsibility to figure out how their research can fit in," says Hudson.
There is still hope for Kelley. NINDS' advisory council will meet this month, and it can make an executive decision to reach below its 10th-percentile cut-off and fund her grant. Kelley will be anxiously awaiting a post-meeting call from Mitler, who will be in the room. And even if the answer is no, Kelley says she is far from giving up. "I don't seem to be crushed. Nothing is going to keep me from doing science," she says. Kelley says that if she could go back and talk to herself as an 11 year old, reading about Nobel prizewinners, she would give nothing but encouragement. "Even if you have to go through this horrible struggle," she says, "it's so much fun that there's just no imagining anything else you could do."
Cutting costs
On 17 October 2008, Rafael-Fortney was in the midst of a grim task: killing two lines of her knockout mice. Two days earlier, she had heard that both her last-chance R01 applications had been returned unscored. Now in her 14th month of bridge funding, the $1,000 in monthly maintenance costs for the mice was no longer tenable. Some 100 of them had to be dispatched.
The comments on both her R01 rejections had been hard to swallow. Despite having added preliminary results for her CASK knockout mouse, the reviewers still complained that she didn't have the other mouse — the DLG knockout — in hand. And the reviewers on the dual PI grant considered the bid to make a claudin-5 knockout a risk too, even though, in earlier rounds, they had expressed great confidence in her ability to do so. Part of the problem, Rafael-Fortney thinks, is that she occupies a difficult middle ground in her scientific career: not senior enough to have years of resources and networks to fall back on in hard times, nor 'young' enough — ten years or closer to her PhD — to profit from NIH programmes targeted at the newest scientists. "Mostly the superstars are getting funded," she says. One NIH administrator had told her: "Unfortunately you're not a giant in the field and you're not at Harvard."
After receiving Rafael-Fortney's desperate e-mail and talking to her at length, her department chair Ostrowski helped her regroup. She has submitted new grants to the American Heart Association and the Muscular Dystrophy Association. At the same time, she has rewritten the failed R01 grant application, stripping it of half of its experiments and adding new ones to qualify it as an entirely fresh application, this time focused on the role of CASK in developing adult skeletal muscle. Events on the national stage could now work in her favour. President Barack Obama's budget request for 2010, due for release in March, and a 2009 budget now being finalized by Congress, could provide a new influx of funds for the NIH. And the economic stimulus bill being discussed by US lawmakers could provide more. But this time around, Rafael-Fortney will only have two chances to apply: in January 2009, the NIH implemented rules that R01 applications can only be submitted twice.
ADVERTISEMENT
But Rafael-Fortney says that she can't give up. "It would mean giving up what I was passionate about, what I have been passionate about my whole life," she says. And she is also unwilling to compromise her scientific aims by proposing less ambitious projects. Recently, she finished reviewing a stack of fellowship applications for the NIH and she says she was tempted to favour the 'safer' proposal, the one with all the preliminary data in hand. "It's really hard to not go for the one experiment that's almost done," she says. Her fear is that if she starts rejecting risky projects, as hers were rejected, then more researchers in her position will be shut out. "It's going to just wipe out the whole middle," she says. "Everyone between the ages of 35 and 50 are just going to be gone in science."
See Editorial.
Updated:
On 10 February 2009, Darcy Kelley learned that her third and final application for an R01 renewal will be awarded funding by the National Institute of Neurological Disorders and Stroke (NINDS). The same grant had previously been rejected for falling below the 10th percentile. Story Landis, director of the institute, made a decision to move the institute’s funding cut-off for grant applications to the 11th percentile after a re-evaluation of the institute’s funding obligations. The change meant that Kelley — and another 7 investigators whose applications had fallen between the 10th and 11th percentile in two recent funding rounds — got funded. Small readjustments to funding cut-offs often occur at NIH institutes as the staff monitor how much money is being spent and how much is left. For instance, if enrolment in a costly clinical trial is slower than expected, then spending on the trial in that financial year is reduced, freeing up funds that can be directed to other grants. “The general strategy is that we start off the fiscal year conservatively, setting the payline at a level we are pretty sure we can afford,â€* says Robert Finkelstein, director of the extramural division at NINDS. As the fiscal year progresses, “we are able to get a little bolderâ€*, he says.
-
References
- Hanft, L. M., Rybakova, I. N., Patel, J. R., Rafael-Fortney, J. A. & Ervasti, J. M. Proc. Natl Acad. Sci. USA 103, 5385–5390 (2006). | Article | PubMed | ChemPort |
- Yang, E.-J., Nasipak, B. T. & Kelley, D. B. Proc. Natl Acad. Sci. USA 104, 2477–2482 (2007). | Article | PubMed | ChemPort |
- Deconinck, A. E. et al. Cell 90, 717–727 (1997). | Article | PubMed | ISI | ChemPort |
- Kelley, D. B. Science 207, 553–555 (1980). | Article | PubMed | ISI | ChemPort |
- Sassoon, D. A, Gray, G. E. & Kelley, D. B. J. Neurosci. 7, 3198–3206 (1987). | PubMed | ChemPort |
- Tobias, M. L. & Kelley, D. B. J. Neurosci. 7, 3191–3197 (1987). | PubMed | ChemPort |
- Zornik, E. & Kelley, D. B. J. Neurosci. 28, 612–621 (2008). | Article | PubMed | ChemPort |
- Sanford, J. L. et al. J. Mol. Cell. Cardiol. 38, 323–332 (2005). | Article | PubMed | ChemPort |
- Mays, T. A. et al. J. Mol. Cell. Cardiol. 45, 81–87 (2008). | Article | PubMed | ChemPort |

