 Embryo development is a game of two halves© Development
Embryo development is a game of two halves© DevelopmentThe road from egg to mouse is a long and tortuous one, but even the first two cells in the embryo have different fates, researchers now report. The cell pair that generate an entire body hold secrets stem cell fans dream of.
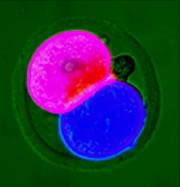
Painting on fluorescent dyes dissolved in olive oil, UK researchers coloured the first two cells of the mouse embryo - one red, one blue. They then tracked the cells as they divided to make a ball of 32-64 cells, the blastocyst.
They found that one cell tends to produce those that make up the embryo body, including future blood, bone and nerve cells. The other gives rise to tissues that nourish the embryo, such as parts of the placenta1. The finding was "very striking", says group leader Magdalena Zernicka-Goetz of the University of Cambridge, UK.
These results are "the first confirmation", that mammalian cell fates are decided so early, says embryologist Richard Gardner of the University of Oxford, UK. Until recently, the young embryo was thought to be a uniform mass of cells; our view is now undergoing something of a revolution.
Early embryonic cells are renowned for their flexibility. If part of a very young embryo is destroyed, the remaining cells can compensate. They are said to be 'totipotent' - able to give rise to all the different cell types in the body. Early decisions that narrow down a cell's fate present an apparent paradox.
But their totipotency does not stop these first cells being predisposed to one destiny, thinks Zernicka-Goetz. Although the cells follow guidelines, if the embryo should be damaged they "can start development [again] from the beginning," she explains.
Why cells specialize so early remains unknown. By keeping their developmental options open, embryos may be better able to survive damage, speculates stem-cell expert Roger Pedersen, also of Cambridge. "It certainly wasn't devised to entertain embryologists," he says.
Potent powers
Mimicking totipotency is the holy grail of stem-cell research. If the underlying molecular signals can be identified, they might be used to transform older cells and tissues, which do not normally repair themselves. Studies such as Zernicka-Goetz's "begin to provide clues", says Pedersen.
The earliest embryonic stages "are very special", he adds, as cells can organize themselves into a complete embryo. Mouse embryonic stem cells used in experiments can be grown in culture only once they have reached the later, blastocyst stage. By this point, they have already lost the ability to self-organize and only form embryos if injected into an existing blastocyst.
On a more fundamental level, such research helps to explain how an embryo sets up the front, back, head and tail of its future body. Earlier this year, Zernicka-Goetz proposed that the point at which the sperm enters the egg may be important in setting up the early axes of the embryo, possibly by altering the cells' internal skeleton2.
-
References
