Abstract
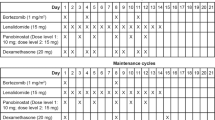
This phase 2 trial evaluated three low-dose intensity subcutaneous bortezomib-based treatments in patients ⩾75 years with newly diagnosed multiple myeloma (MM). Patients received subcutaneous bortezomib plus oral prednisone (VP, N=51) or VP plus cyclophosphamide (VCP, N=51) or VP plus melphalan (VMP, N=50), followed by bortezomib maintenance, and half of the patients were frail. Response rate was 64% with VP, 67% with VCP and 86% with VMP, and very good partial response rate or better was 26%, 28.5% and 49%, respectively. Median progression-free survival was 14.0, 15.2 and 17.1 months, and 2-year OS was 60%, 70% and 76% in VP, VCP, VMP, respectively. At least one drug-related grade ⩾3 non-hematologic adverse event (AE) occurred in 22% of VP, 37% of VCP and 33% of VMP patients; the discontinuation rate for AEs was 12%, 14% and 20%, and the 6-month rate of toxicity-related deaths was 4%, 4% and 8%, respectively. The most common grade ⩾3 AEs included infections (8–20%), and constitutional (10–14%) and cardiovascular events (4–12%); peripheral neuropathy was limited (4–6%). Bortezomib maintenance was effective and feasible. VP, VCP and VMP regimens demonstrated no substantial difference. Yet, toxicity was higher with VMP, suggesting that a two-drug combination followed by maintenance should be preferred in frail patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008; 111: 2516–2520.
Brenner H, Gondos A, Pulte D . Expected long-term survival of patients diagnosed with multiple myeloma in 2006–2010. Haematologica 2009; 94: 270–275.
Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W et al (eds). SEER Cancer Statistics Review, 1975–2007. Available at: http://seer.cancer.gov/csr/1975_2007/S (last accessed 10 July 2015).
Palumbo A, Bringhen S, Mateos MV, Larocca A, Facon T, Kumar SK et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood 2015; 125: 2068–2074.
Bringhen S, Mateos MV, Zweegman S, Larocca A, Falcone AP, Oriol A et al. Age and organ damage correlate with poor survival in myeloma patients: meta-analysis of 1435 individual patient data from 4 randomized trials. Haematologica 2013; 98: 980–987.
San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med 2008; 359: 906–917.
Fayers PM, Palumbo A, Hulin C, Waage A, Wijermans P, Beksaç M et al. Thalidomide for previously untreated elderly patients with multiple myeloma: meta-analysis of 1685 individual patient data from 6 randomized clinical trials. Blood 2011; 118: 1239–1247.
San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M et al. Continued overall survival benefit after 5 years' follow-up with bortezomib-melphalan-prednisone (VMP) versus melphalan-prednisone (MP) in patients with previously untreated multiple myeloma, and no increased risk of second primary malignancies: final results of the Phase 3 VISTA Trial [abstract]. Blood 2011; 118: (abstract 476).
Mateos MV, Richardson PG, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O et al. Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: updated follow-up and impact of subsequent therapy in the phase III VISTA trial. J Clin Oncol 2010; 28: 2259–2266.
Wijermans P, Schaafsma M, Termorshuizen F, Ammerlaan R, Wittebol S, Sinnige H et al. Phase III study of the value of thalidomide added to melphalan plus prednisone in elderly patients with newly diagnosed multiple myeloma: the HOVON 49 Study. J Clin Oncol 2010; 28: 3160–3166.
Hulin C, Facon T, Rodon P, Pegourie B, Benboubker L, Doyen C et al. Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma: IFM 01/01 trial. J Clin Oncol 2009; 27: 3664–3670.
Niesvizky R, Flinn IW, Rifkin R, Gabrail N, Charu V, Clowney B et al. Community-based phase IIIB trial of three UPFRONT bortezomib-based myeloma regimens. J Clin Oncol 2015; 33: 3921–3929.
Kumar S, Flinn I, Richardson PG, Hari P, Callander N, Noga SJ et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood 2012; 119: 4375–4382.
Facon T, Mary JY, Pégourie B, Attal M, Renaud M, Sadoun A et al. Dexamethasone-based regimens versus melphalan–prednisone for elderly multiple myeloma patients ineligible for high dose therapy. Blood 2006; 107: 1292–1298.
Durie BG, Kyle RA, Belch A, Bensinger W, Blade J, Boccadoro M et al. Myeloma management guidelines: a consensus report from the Scientific Advisors of the International Myeloma Foundation. Hematol J 2003; 4: 379–398.
Kyle RA, Rajkumar SV . Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia 2009; 23: 3–9.
Lawton MP . Scales to measure competence in everyday activities. Psychopharmacol Bull 1988; 24: 609–614.
Charlson ME, Pompei P, Ales KL, MacKenzie CR . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383.
National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), version 3.0. 2006. Available at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf(accessed 3 January 2015).
Simon R . Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989; 10: 1–10.
Kaplan EL, Meier P . Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Mateos MV, Oriol A, Martínez-López J, Gutiérrez N, Teruel AI, de Paz R et al. Bortezomib, melphalan, and prednisone versus bortezomib, thalidomide, and prednisone as induction therapy followed by maintenance treatment with bortezomib and thalidomide versus bortezomib and prednisone in elderly patients with untreated multiple myeloma: a randomised trial. Lancet Oncol 2010; 11: 934–941.
Palumbo A, Bringhen S, Rossi D, Cavalli M, Larocca A, Ria R et al. Bortezomib–melphalan–prednisone–thalidomide followed by maintenance with bortezomib–thalidomide compared with bortezomib–melphalan–prednisone for initial treatment of multiple myeloma: a randomized controlled trial. J Clin Oncol 2010; 28: 5101–5109.
Mateos MV, Oriol A, Martínez-López J, Gutiérrez N, Teruel AI, López de la Guía A et al. Maintenance therapy with bortezomib plus thalidomide or bortezomib plus prednisone in elderly multiple myeloma patients included in the GEM2005MAS65 trial. Blood 2012; 120: 2581–2588.
Palumbo A, Hajek R, Delforge M, Kropff M, Petrucci MT, Catalano J et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med 2012; 366: 1759–1769.
Acknowledgements
We thank all the patients who participated in the study, the nurses Rosalia Capobianco and Giacomo Castorina, the data managers Giulia Lupparelli, Marta Santoro and the editorial assistant Giorgio Schirripa.
Author contributions
AL, MB, PS, and AP designed the study and supervised its conduct and the data analysis. AL, SB, MTP, SO, APF, TC, OV, GB, AML, FM, VM, RP, LDR, PO, IDV, SS, AMC, EP, DD, MG, TG, CN, EA, LDP, CC, CM and MO recruited patients in the source studies and/or provided relevant data. AL collected and assembled the data. RP and SS performed the statistical analysis. AL and AP analyzed and interpreted the data. AL and AP drafted the initial manuscript. All authors were given unrestricted access to the data, critically reviewed the manuscript drafts, approved the final version and made the decision to submit it for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
AL has received honoraria from Celgene and Jannsen-Cilag. SB has received honoraria from Celgene, Janssen-Cilag and served on the advisory committee for Mundipharma. MTP has received honoraria from Celgene, Janssen-Cilag, Mundipharma, Sanofi, Amgen and Bristol-Myers Squibb. TC has received honoraria from Celgene, J&J, Amgen and Bristol-Myers Squibb. TG has received research funding from Celgene. CN has received honoraria from Celgene, Janssen-Cilag and Mundipharma. MO has received honoraria from Janssen-Cilag. MB has received consultancy fees from and served on the scientific advisory board for Jannsen-Cilag, Sanofi, Amgen and Celgene. PS has received research support from Onyx, Janssen, Celgene, Millennium and served on the advisory board for Onyx, Janssen, Celgene and Millennium. AP has received consultancy fees, research funding and honoraria from Jannsen-Cilag.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Larocca, A., Bringhen, S., Petrucci, M. et al. A phase 2 study of three low-dose intensity subcutaneous bortezomib regimens in elderly frail patients with untreated multiple myeloma. Leukemia 30, 1320–1326 (2016). https://doi.org/10.1038/leu.2016.36
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2016.36
This article is cited by
-
The prevalence and outcomes of frail older adults in clinical trials in multiple myeloma: A systematic review
Blood Cancer Journal (2023)
-
Advantages of multi-arm non-randomised sequentially allocated cohort designs for Phase II oncology trials
British Journal of Cancer (2022)
-
Octogenarian newly diagnosed multiple myeloma patients without geriatric impairments: the role of age >80 in the IMWG frailty score
Blood Cancer Journal (2021)
-
Rare case presentation: DLBCL and plasma cell myeloma coexisting together
Comparative Clinical Pathology (2020)
-
Subcutaneous versus Intravenous Bortezomib Administration for Multiple Myeloma Patients: a Meta-analysis
Current Medical Science (2018)