The radical plan for vaccine equity
Charity failed to provide adequate vaccines for the global south. Now, 15 countries are seeing whether an open-science model can end a dangerous legacy of dependency.
Travel for this article was supported by the Pulitzer Center.
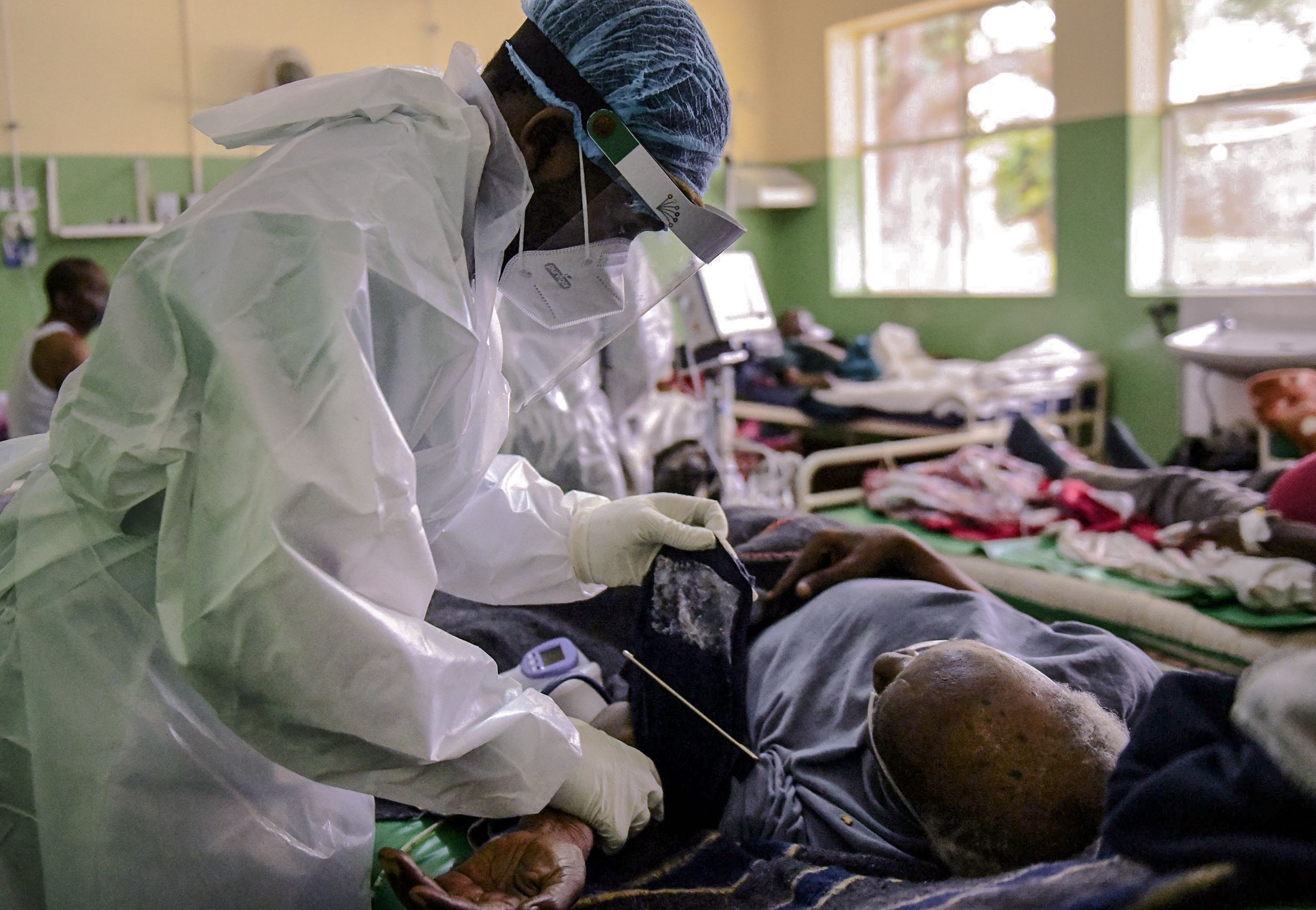
An invisible divide formed early last year as COVID-19 vaccines spread through rich countries, while the rest of the world waited. In one part of the globe, newly vaccinated doctors and nurses breathed sighs of relief and grandparents hugged their grandchildren for the first time in months. In the other part, hospitals overflowed with an unmitigated surge of COVID-19.
“We saw our fellow nurses dying with COVID,” says Milly Kumwenda, a nurse at Queen Elizabeth Central Hospital in the city of Blantyre in southern Malawi, as she recalls a deadly surge of the disease in January 2021. After two cabinet ministers died of COVID-19, Malawi’s president declared a state of national disaster. The aid agency Médecins sans Frontières (MSF, also known as Doctors without Borders) rushed to help and issued an appeal to the rest of the world: “Malawi urgently needs access to the vaccine.”
Vanishingly few doses arrived — in unpredictable spurts and often close to expiry. By the time the next surge hit in July 2021, just 1% of Malawians had been vaccinated. Many people had stopped seeking care by then because they had lost faith in the health system, says Loveness Gona, another nurse at the hospital. There are few ventilators in Malawi, no antiviral infusions or monoclonal antibody treatments, and chronic shortages of drugs to manage deadly symptoms such as blood clots and inflammation. These are some of the reasons that death rates among people hospitalized for COVID-19 in low-income countries have been more than twice as high as in wealthy nations1. Gona remembers coming into work to find corpses propped up in chairs in the hospital waiting room, their loved ones demanding a test. “Somewhere else, they’d be alive,” she says.

Vast, ongoing delays in the global distribution of COVID-19 vaccines have resulted in death on a massive scale and arguably allowed the evolution of the Omicron variant, which was first reported in South Africa late last year. Such inequities are jarring, but hardly new. Many years passed before life-saving vaccines and drugs for pneumonia and HIV were widely available in Africa, and important treatments for cancer and cystic fibrosis that are common in rich countries remain almost unobtainable in poorer ones.
At the root of the problem lies a dependence on the limited goodwill of countries — mainly in the global north — where the majority of large pharmaceutical companies are based. That’s why more than a dozen countries in the global south are banding together with the World Health Organization (WHO) and other groups in a long-term initiative to build vaccine- and drug-making capacity throughout Africa, South America, Asia and Eastern Europe. “The COVID-19 pandemic has shown that reliance on a few companies to supply global public goods is limiting and dangerous,” said WHO director-general Tedros Adhanom Ghebreyesus as he announced the initiative last year.
Called the mRNA vaccine technology transfer hub, the initiative is built around the shiny new promise of messenger RNA as a tool for vaccines and drugs. At the hub’s core is a small biotechnology firm in Cape Town, South Africa, called Afrigen Biologics and Vaccines. It is linked to South African universities and pharmaceutical companies based in 15 countries, including Senegal, Argentina and Indonesia (see ‘Changing the equation’). Together, these groups aim to make their own effective mRNA vaccine against COVID-19, before expanding into other diseases that are relevant to their regions, be it HIV, Zika or measles.
Beyond technical difficulties, the initiative faces economic policies and a geopolitical legacy tilted in favour of the global north. This includes a dense web of mRNA vaccine patents that could deter companies involved with the hub that want to sell their products. The hub will also need to convince governments and international agencies to purchase doses from local manufacturers — even if they are initially more expensive than those produced by big pharma.
Still, proponents laud the hub’s revolutionary collaborative approach, and argue that it has the potential to prevent the next pandemic by making every region of the world more self-reliant. “Until you can vaccinate the whole world in six months instead of six years, we’re going to continue with challenges like we are having right now with the variants,” says Barney Graham, a researcher who conducted foundational work on mRNA vaccines at the US National Institutes of Health (NIH) in Bethesda, Maryland, and who is one of the hub’s advisers.
Some see a more fundamental goal — to lessen many countries’ dependence on wealthy nations by fostering skilled employment and economic growth. For all these reasons, Petro Terblanche, Afrigen’s straight-talking managing director, arrives at her office as the Sun rises each day. “This is the most complex project that I’ve ever taken on, but it’s also the most inspiring and motivating one,” she says. “If we do not, on this continent, establish our ability to create essential medicines and vaccines, we will forever sit in this inequality situation.”
“I am not risk-averse.”
Petro Terblanche
Patent problems
On a misty April morning, Terblanche helps herself to another cup of coffee from the machine in Afrigen’s kitchenette. She has a small window of time before she’ll meet a team of scientists visiting from Biofarma, a government-owned vaccine company in Bandung, Indonesia. The scientists have come at the behest of the Indonesian ministry of health, which hopes to eventually make mRNA vaccines against dengue fever and other killer diseases in southeast Asia. For similar reasons, groups from Argentina and Brazil came to learn from Afrigen a few weeks earlier.
In addition to managing this rotation, Terblanche is navigating an ever-shifting set of conflicts and collaborations at the hub. Initially, the WHO hoped that one of the various companies that developed the leading mRNA vaccines in use today — Moderna, based in Cambridge, Massachusetts; Pfizer, in New York City; or BioNTech, in Mainz, Germany — would assist Afrigen and allow the hub’s network of companies to sell mRNA vaccines, at least in low- and middle-income countries. When they didn’t respond, the WHO announced that the hub would reproduce Moderna’s vaccine from publicly available information.
The companies’ recalcitrance didn’t surprise Terblanche, who has led biotechnology projects for some 30 years. “Imagine you’re Moderna: mRNA is your baby, and you are making $1,000 per second in profit on it,” she says. “Why would you let this technology go to an open-source kind of model?”
Instead, she convinced her team that the challenge represented an opportunity to improve on the Moderna shot. In the most literal sense, she is drawn to obstacles: she jumped horses over hurdles for years before falling beneath the hooves of a mare. Cracking a slight smile, she says, “I am not risk-averse.”
In March, Afrigen turned heads when it announced that it had succeeded in producing a COVID-19 vaccine similar to the one made by Moderna — despite warnings from the pharmaceutical industry that mRNA technology was too new and complex for alternative companies to cook up. Now, the team is testing the efficacy of this candidate in mice, while constructing laboratories that meet the strict safety standards required for manufacturing human vaccines. Giving a tour of the facilities, Afrigen’s research technologist, Emile Hendricks, enters negative-pressure rooms designed to stop microorganisms from escaping and points out a futuristic-looking instrument that makes lipid nanoparticles to protect the vaccine mRNA as it’s delivered into the body. As soon as one of the hub’s scientists spotted the equipment’s make and model in a news clip from a CNN television special on Pfizer’s vaccine efforts, Afrigen placed an order.

Once the new facilities are approved by South African regulators — hopefully by early next year — Afrigen will make the mRNA vaccines needed for small safety studies in people. At the same time, production will move to another, larger biopharmaceutical company in Cape Town called Biovac, which will conduct clinical trials to test the vaccine’s efficacy and apply for authorization from regulatory bodies. The 14 international companies visiting Afrigen will scale up production, run studies and seek regulatory authorization, too, as well as explore how to make the vaccine cheaper and more stable at higher temperatures, so that it is more practical for countries without reliable electricity for deep-freezing.
But most of all, researchers involved with the hub are searching for an mRNA vaccine that won’t infringe on intellectual property (IP) held by Moderna and other companies. Of particular concern when I visited Afrigen last April was one of the four lipids used in current mRNA vaccines, called SM-102. The lipid has been at the centre of heated lawsuits between Moderna and Arbutus Biopharma, a Canadian company that conducted research on lipid nanoparticles with the University of British Columbia in Vancouver. Such tangled webs make it hard for the hub to even know whose permission it needs to produce a vaccine. This duty falls on the group co-leading the hub, the United Nations-backed Medicines Patent Pool, which seeks licences to use patented technologies, or finds substitutes.
While in the research stage, the hub’s work is legal. Patents come into play only once a company tries to sell a product. But ownership issues were causing consternation among the team visiting from Indonesia. “Maybe we can use another lipid?” says Ryan Adibagus Haryanto, a formulation researcher at Biofarma, who is sitting with his co-workers around a long table in Afrigen’s conference room. His colleague Latri Rahmah counters with a sigh, “If we use another one, it will be more time.” A researcher beside her warns in a soft voice, “We would have to start again from zero.”
The Indonesian researchers conclude that they must push on. “High-income countries have all of the new technology, but low- and middle-income countries need it, too,” Biofarma biochemist Anna Sanawati tells me. “We don’t know how this pandemic will change with new variants, and if there is another pandemic, we need to be able to prepare our own weapons to defend us from a new disease.”

Unmasking inequality
South Africa was not an obvious home for the hub. When the WHO announced the programme in June 2021, the need for COVID-19 vaccines was acute — hundreds of thousands of people were dying in countries desperate for jabs. India, Brazil or another country that regularly produces other vaccines used in the global south seemed like a more realistic base for operations, because starting from scratch takes time. But the WHO’s chief scientist, Soumya Swaminathan, defended the decision by explaining that the programme was less about filling the immediate need for COVID-19 vaccines, and more about a long-term vision of establishing pharmaceutical capability in every region of the globe. That deficiency is most acute on the African continent, which produces just 1% of all of the vaccines it consumes.
With Africa as a priority, South Africa won the bid as the hub’s core because its application promised collaboration between the government’s medical research agency, existing biotechnology companies and universities with experience in mRNA production. In addition, the WHO picked companies in five other African countries — Egypt, Tunisia, Kenya, Nigeria and Senegal — along with those from nine countries on other continents to collaborate with and learn from Afrigen. The hope is that each of these places will supply vaccines to their respective regions, reaching countries that have been last in line during this pandemic. Over the course of this year, South Africa, India and other middle-income countries have significantly boosted their vaccination rates, but barely 10% of people in nations such as Malawi, the Democratic Republic of the Congo and South Sudan have received a single shot (see ‘Protection divide’ and ‘How deadly is COVID-19?’).
Over tea at an outdoor restaurant in Blantyre, Kondwani Jambo, an immunologist at the Malawi-Liverpool-Wellcome Clinical Research Programme based in the city, says, “The inequality of COVID vaccines just reveals the vast inequalities that exist.” He explains how the country’s lack of jabs unmasks an unfairness that pervades all aspects of health care. The problems are multi-layered. “We are facing four pandemics — HIV, malaria, tuberculosis and COVID — and on top of that we have a level of poverty that people die from.”
In rural towns outside Blantyre, brown fields of maize stretch to the mountains on the horizon. Chief Harrison Chauluka of Mkunda village gestures at rotted cobs on the stalk as he says that erratic rains due to climate change have killed crops, and people don’t have enough to eat. His concerns are backed by scientific models predicting that climate change will take some of its most devastating tolls on agriculture-dependent, low-income communities2. Trade disruptions during the pandemic have exacerbated the problem, adds Chauluka, driving up the cost of imported goods, including fertilizer.
Conversations with other Malawians in neighbouring agrarian towns all come back to how hardships have multiplied during the pandemic. Waiting to be seen by nurses at a small clinic near Mkunda, Patricia Mangala, an older woman in a loose, mint-green blazer, says she’s upset that the pandemic has come at a time when she’s physically weak from malnourishment. This slows down her farming, as does the pain that she’s felt in her heart since being hospitalized with COVID-19 in January 2021. She walked for two hours to reach the clinic today, but the nurses have no way to treat her heart problem — and so Mangala leaves with only her monthly doses of HIV medicines. Later, Ellen Msukwa, a nurse at the clinic, tells me that many of the HIV patients aren’t faring well because their medicines must be taken with food, which is scarce.
But the clinic can’t monitor their conditions because it is out of tests to assess HIV levels. It also lacks antibiotics used to treat common infections among immunocompromised people. If medical supplies were made nearby in South Africa, Msukwa speculates, they would have fewer shortages and people would be more inclined to trust that clinics could help them.
Many researchers agree with her assessment. Supplies alone won’t solve health disparities, but they are an essential part of the solution. “What is clear is that having this manufacturing capacity is actually really important to the health systems of these regions,” says Andrea Taylor, a global health researcher and former head of a COVID-19 vaccine distribution study at Duke University in Durham, North Carolina. “This goes beyond the acute need for COVID-19 right now. It is really critical.”
“If given a chance, I would get the vaccine,” says Charles Bokosi, a a 50-year-old man in southern Malawi who says he worries about COVID-19 because his immune system is weakened from HIV. If COVID-19 vaccines were made nearby in South Africa, Bokosi believes they would have prevented many deaths in 2021.
COVID-19 vaccines aren’t the only treatments lacking in low-income countries. Common drugs, including antibiotics, are often in short supply. “It’s difficult as a health worker because we wish everyone could receive the quality care that they are supposed to receive,” says Ellen Msukwa, a nurse at Namadzi Health Centre in Malawi.
“I am strong,” says Patricia Mangala, a 62-year-old woman who was hospitalized for COVID-19 last year. “God watches over me.” Ever since having COVID-19, Mangala has felt pain in her chest but she has no access to treatment for this or her high blood pressure. She was vaccinated against COVID-19 earlier this year and now encourages others to do the same.
“Many people in this village wanted the shot, but when they went to get it, the vaccine was not available,” says Harrison Chauluka, the chief of Mkanda village. He welcomes the idea of African vaccine-manufacturing plants so that Malawians need not rely on the goodwill of rich countries.
Problems linked to the pandemic layer on top of other dire issues. Crops have failed across southern Malawi because of erratic weather coupled with the rising cost of fertilizer. The resulting food insecurity has rendered many people more vulnerable to disease.
Information monopoly
In the 1980s, pharmaceutical companies based in the United States led a push to expand IP rights globally at a time when the firms faced potential competition from a growing generic-drug industry in India and Brazil3. Leading economists warned that restrictive policies would impede access to life-saving medicines, but in 1995, the TRIPS Agreement came into effect. This meant that more than 150 countries belonging to the World Trade Organization (WTO) must adhere to particular IP rules, such as respecting patents for a minimum of 20 years. Joseph Stiglitz, a Nobel-prizewinning economist at Columbia University in New York City, called the agreement “theoretically indefensible and ethically unacceptable”, arguing that it serves corporate interests in developed countries, often at the expense of the health of the world’s poor4.
Widespread anger over the TRIPS Agreement erupted as the HIV epidemic escalated in the late 1990s and early 2000s. With a huge HIV-infected population and little access to expensive antiretroviral drugs, South Africa proposed a bill to allow the use of more-affordable generic medicines. This arguably ran counter to TRIPS and, in response, Pfizer and other pharmaceutical companies threatened Nelson Mandela, the president at the time, with a lawsuit. The United States proposed sanctions. Neither materialized because of public outcry, but hundreds of thousands of people died in Africa during the years when HIV drugs existed but were unavailable on the continent. This period is seared into the memories of many. “The biggest business back then was coffin-making,” recalls Charlie Masiku, director of the COVID-19-response team at MSF in Blantyre. “We lost so many people who were just coming up in their 30s, people in the press, in politics, civil servants.” When COVID-19 hit, Masiku knew the country would be abandoned again.
SARS-CoV-2
It has taken more than a year for COVID-19 vaccines to reach 20% of the population in low-income nations, which wealthy countries achieved in April 2021.
Haemophilus influenzae (a cause of pneumonia)
Such delays are not special to this pandemic. Decades passed, for example, before a Haemophilus influenzae vaccine was widely available in lower-income countries.
Streptococcus pneumoniae
And a vaccine to prevent pneumococcal disease caused by Streptococcus pneumoniae remains much less available in low- and middle-income countries.
HIV/AIDS
Many people in lower-income countries died of HIV/AIDS during the years when antiretroviral therapy was inaccessible in those nations*.
* The higher-income classification is excluded owing to insufficient data from rich countries such as the United States.
Sources: Our World in Data (SARS-CoV-2 and HIV)/UNICEF (H. influenzae and S. pneumoniae; see https://go.nature.com/3NMTQNJ)
Masiku wasn’t alone. Predicting the unfair distribution of COVID-19 vaccines, South Africa and India put forward a proposal to waive TRIPS in October 2020. In the following months, many countries and international organizations, including the United States and the WHO, backed a waiver. But the proposal languished at the WTO for around 20 months, with the European Union and others blocking the measure. Opponents argued that it would undermine innovation and do little to fill the gap in vaccine supplies. On 17 June this year, the WTO passed a heavily revised version of the waiver that offers a temporary relief on some restrictions. The deal was widely criticized as too compromised to make a difference to most manufacturers in the global south, including the companies involved with the WHO’s mRNA hub.
In the meantime, the WHO has continued to press Moderna to license its IP to the hub, or at least share its data, such as the details of clinical trials, that could help Afrigen to confirm that its vaccine candidate meets similar metrics. On 28 April, Tedros made a case for these measures at a meeting with Moderna’s shareholders, saying that the hub could have a vaccine ready one year earlier if Moderna worked with them. Since then, nearly 30% of Moderna’s shareholders, including its directors and senior executives, have endorsed this plea, according to the non-profit organization Oxfam, based in Nairobi, Kenya, which submitted the resolution to Moderna. But the company seems to be moving in the opposite direction — in ways that might undermine the hub’s work.
Soon after Afrigen announced that it would attempt to make a Moderna-like vaccine, Moderna obtained several vaccine patents in South Africa that won’t expire until 2034. One of the claims, which covers the method of mRNA vaccine production broadly, was rejected or withdrawn in Canada, Israel, Singapore and South Korea. This patent could potentially frustrate the hub’s attempts to make and sell mRNA vaccines for any disease, says Zain Rizvi, a researcher specializing in access to medicines at the advocacy organization Public Citizen, based in Washington DC. Such actions have drawn condemnation from researchers who see them as an abuse of a patent system designed to reward companies fairly for their investment into research and development. Moderna’s private investments have been more than paid off, because its costs were offset by public funding, according to a Public Citizen report. Much of the foundational work on mRNA vaccines was conducted at universities and at the NIH, and the US government gave the company roughly US$1 billion to underwrite its clinical trials. Moderna subsequently reported $18 billion in total revenue in 2021 and has forecast $19 billion this year. Thanks to its vaccine sales, two Moderna founders and one investor are newly minted billionaires.
“It is really hard to watch the profiteering we have seen in the middle of the pandemic,” says Larry Brilliant, an epidemiologist who helped to eradicate smallpox through vaccination campaigns. “I don’t think history will be kind to those who had life-saving interventions and made the decision to maximize profit instead.”
Moderna did not respond to requests from Nature for comment. But in an interview with The Wall Street Journal, Moderna’s chief executive Stéphane Bancel said that the company won’t impede Afrigen’s work in South Africa; he made no mention of the 15 larger companies working with the hub. He added, “I don’t understand why, once we’re in an endemic setting when there’s plenty of vaccine and there’s no issue to supply vaccines, why we should not get rewarded for the things we invented.”
Uncertain about Moderna’s position, Afrigen announced a collaboration with the NIH on the development of next-generation mRNA vaccines and therapeutics on 8 July, and Terblanche is discussing potential partnerships with six biotechnology companies. Meanwhile, academic researchers affiliated with the hub are exploring different mRNA sequences and lipids that might make Afrigen’s vaccine candidate sufficiently distinct from Moderna’s. “It’s like reinventing the wheel,” confesses Abdullah Ely, a molecular biologist at the University of the Witwatersrand in Johannesburg, South Africa.
Despite the hub’s efforts, next-generation mRNA vaccines might still be entangled in patent thickets if some components of the technology have been claimed by others. An astounding number of patents — estimated at more than 80 — surround the mRNA vaccines, according to one analysis5. A thorny IP landscape isn’t as daunting for big companies with the capital to litigate, explains Tahir Amin, a lawyer and co-founder of the Initiative for Medicines, Access & Knowledge (I-MAK), a non-profit group based in New York City. Amin says that the hub could boldly move forward, too, and harness public condemnation if Moderna or other companies file a lawsuit. But this option is off the table because the Medicines Patent Pool vows not to infringe on patents. Indeed, the agency’s model relies on persuading pharmaceutical companies to voluntarily license their technologies to alternative manufacturers, often in exchange for royalty fees.
Recalling the years of fighting required to get HIV drugs to southern Africa, Fatima Hassan, a human-rights lawyer who now heads the Health Justice Initiative in Cape Town, predicts that this cautious approach could backfire. She says that radical policy changes, such as government action to override patent barriers, could be required for the hub to succeed. And she worries that the WHO and the Medicines Patent Pool won’t push back hard enough against Moderna if it back-pedals on its promises not to interfere with the hub’s work. Sitting in a windswept park beside the Atlantic Ocean in Cape Town, Hassan raises her voice to ask, “What is the plan B?”
“Everyone is waiting on benevolence and volunteerism. But, hello, people are dying! We must take the necessary actions to save lives and push for the transfer of technology.”
Fatima Hassan
Credit: Barry Christianson
Market force
Another obstacle for the hub is that locally manufactured vaccines might initially cost more than those made by established, multinational companies that produce them at greater scales. Furthermore, the price difference might be even more drastic if these firms drop their prices far below what a young company can afford. “This happens,” says Mark Dybul, a global-health researcher at Georgetown University in Washington DC and a former director of the Global Fund to Fight AIDS, Tuberculosis and Malaria. “If you’re massive and have billions of dollars, you can take people out of the market.”
These fears could be put to rest by contracts to buy local vaccines, similar to the advanced purchasing agreements the US government signed with Moderna. No one has made such arrangements with the hub, although the South African government has promised to support it. Glaudina Loots, the director of health innovation at South Africa’s Department of Science and Innovation, says that the government would pay a price premium — ”within reason” — because of the many benefits of local production, including the impact on the country’s broader economy (see ‘Where is big pharma concentrated? and ‘Vaccines in Africa’).
However, South Africa’s population represents just 4% of the people in Africa. A much more significant buyer would be the vaccine alliance Gavi, based in Geneva, Switzerland, which purchases vaccines for 40 African countries and dozens of others in the developing world. Gavi has said almost nothing about the hub since its inception. In a response to Nature, a spokesperson writes that regional manufacturing is critical for self-sufficiency in lower-income countries, and that the organization is “well positioned, and deeply committed” to supporting vaccine manufacturing in Africa.
Kate Elder, a vaccine policy adviser for the MSF Access Campaign, says that Gavi’s comments are evasive, and that’s not surprising. Gavi’s board responds to requests from recipient countries and relies on its donors. The responsibility for supporting the hub’s mission, therefore, falls to governments of lower-income countries and to those holding Gavi’s purse strings, such as the United Kingdom, the United States and the Bill & Melinda Gates Foundation in Seattle, Washington. “It is up to regional bodies to make sure the hub survives,” Elder says.
In an ominous sign, the first company in Africa involved in the manufacture of COVID-19 vaccines, Aspen Pharmacare based in Durban, South Africa, is currently in danger of pivoting away from this part of its business. It has been completing the final steps of production of the vaccine from Johnson & Johnson (J&J) in New Brunswick, New Jersey, which is preferred in several African countries because of its stability at cool, rather than freezing, temperatures. Aspen senior executive Stavros Nicolaou had expected orders from COVAX, a key initiative co-led by Gavi, which is responsible for procuring COVID-19 vaccines for lower-income countries. COVAX has purchased millions of J&J doses, but none are from Aspen. “That goes to the heart of the issue,” Nicolaou says of the hub’s prospects. “Unless you get the procurement agencies to commit upfront to ordering from African facilities — unless you can get that upfront — then they’re all going to shut down,” he warns. “So, in the next pandemic, Africa will be at the back of the queue again.”
A Gavi spokesperson says that J&J has not granted its requests to ship doses from Aspen, and that Gavi is not ordering additional doses from Aspen directly because demand has plateaued. J&J did not respond to Nature’s request for comment.
Although vaccine supplies remain inadequate in many African countries, governments have requested a pause in shipments because they can’t use doses fast enough. This is largely because of a lack of funds and support to distribute the vaccines, inform people about them and combat rumours. In towns without paved roads in southern Malawi, for example, several people who have no phones, no televisions and no Internet access tell me they would get the jabs but don’t know where to find them. And without cars or buses, they must walk for hours to reach hospitals that might or might not have the shots on site. Malawi and many low-income countries do have strong childhood vaccination networks that could be channelled for COVID-19 vaccines, but doing that takes a level of organization that’s difficult to manage when supplies are unreliable.
The situation is very different from the one in the United States, where the federal government was assured of steady vaccine batches to be distributed through local and state health departments, hospital networks and pharmacy chains. Jambo points out that even with a huge budget, and all of that lead time and planning, the United States has discarded millions of unused doses. He compares this situation to Malawi’s first large shipment of vaccines, which were donated close to expiry and unexpectedly, just before the Easter holiday in 2021. The government scrambled to send doses around the country, along with syringes and information about where people could be vaccinated. Still, about 20% of 102,000 doses had to be incinerated because they expired before they made it into arms. The international media placed the blame on Malawi, rather than on a broken, charity-based system for getting vaccines around the world, says Jambo. “The news made it sound like ‘we are giving you vaccines, and you are ungrateful’.”

No option
The WHO and many global-health researchers say that the disparities on display during the pandemic underline the need for regional manufacturing. Rather than a show of solidarity, nations that made COVID-19 vaccines protected themselves first. The United States, the United Kingdom and India, for example, essentially blocked exports of COVID-19 vaccines until they had secured ample doses for their populations. But widespread manufacturing capacity can still fail if IP remains tightly held by single companies, or if quality control cannot be roundly assured, warns Andrew Hill, a pharmacology researcher at the University of Liverpool, UK.
When pressed about this and several other obstacles, Patrick Tippoo becomes exasperated: “Yes, it is risky — but do we have an option not to do this?” asks Tippoo, an executive at Biovac, the company tasked with taking the hub’s vaccine to market in South Africa. “What we are trying to do is minimize the vulnerability of many countries, and for that, you’ve got to take a chance!”
He is far from a lone voice. The Africa Centres for Disease Control and Prevention has announced a goal of producing 60% of vaccines used by Africa, in Africa, within the next 20 years. Together with the African Union, the agencies launched the Partnership for African Vaccine Manufacturing, which includes a variety of efforts to scale up pharmaceutical capacity, including the hub and more-conventional efforts, such as a World-Bank-funded project to upgrade vaccine-making facilities at the Pasteur Institute of Dakar in Senegal.
Even if the hub fails in its mission, it is bound to be revealing as a bold experiment testing whether an open-science model can build capacity. This is not without precedent. In the 1960s and 1970s, West Germany, Switzerland and Japan all built up their biotechnology sectors with public funding, and didn’t enforce patents until the industry was mature. “That’s kind of been the development model for how the West got to be where it is, and where China was until recently,” explains Luke McDonagh, an IP researcher at the London School of Economics and Political Science. He and other researchers even predict that this model can result in more innovation through collaboration. “If you no longer have this stranglehold on ideas and funding in the global north, we could be so much further along,” Dybul suggests.
When she is questioned about the hub’s obstacles, Terblanche becomes philosophical. The problems that Afrigen has begun to bump up against are the levers that concentrate power with companies in the global north — the policies and practices that must be changed for a more equitable world. “We need to challenge the idea that this dominance is necessary,” Terblanche tells me. “I thought this was going to be a hell of a difficult technology project when we started,” she says with a laugh. “Now, the technology is the easy part.”
This article is also available as a pdf version.
References
- Levin, A. T. et al. BMJ Glob. Health 7, e008477 (2022).
- Msowoya, K., Madani, K., Davtalab, R., Mirchi, A. & Lund, J. R. Water Res. Mgmt 30, 5299–5312 (2016).
- Drahos, P. & Braithwaite, J. in Balancing Act: Law, Policy and Politics in Globalisation and Global Trade (eds Chen, J. & Walker, G.) 204–223 (Federation Press, 2004).
- Baker, D., Jayadev, A. & Stiglitz, J. Innovation, Intellectual Property, and Development: A Better Set of Approaches for the 21st Century (AccessIBSA, 2017).
- Gaviria, M. & Kilic, B. Nature Biotechnol. 39, 546–548 (2021).
Photography by Samantha Reinders in South Africa and Thoko Chikondi in Malawi, unless otherwise noted.