Abstract
Objective:
Procyanidins are polyphenolic compounds with beneficial effects on health in relation to cardiovascular disease and metabolic syndrome. In this study, we evaluated the potential beneficial effects of low doses of a grape seed procyanidin extract (GSPE) on body weight and fat deposition.
Design:
Four groups of hamsters were fed either a standard diet (STD) or a high-fat diet (HFD) for 30 days and supplemented with either GSPE at 25 mg per kg of body weight per day (STD-GSPE and HFD-GSPE groups) or vehicle (STD and HFD groups) during the last 15 days of the study.
Results:
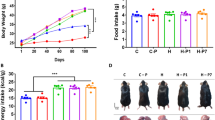
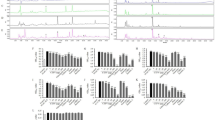
A significant decrease in body weight gain was observed in both GSPE-treated animals at the end of the experiment. GSPE treatment significantly reduced the adiposity index and the weight of all the white adipose tissue depots studied (retroperitoneal (RWAT), mesenteric (MWAT), epididymal (EWAT) and inguinal (IWAT)) in both GSPE-treated groups. GSPE administration reversed the increase in plasma phospholipids induced by the HFD feeding. In the RWAT, GSPE treatment increased the mRNA expression of genes related to β-oxidation and the glycerolipid/free fatty acid (GL/FFA) cycle, mainly in HFD-GSPE animals. In the MWAT, the effects of GSPE at the transcriptional level were not as evident as in the RWAT. Moreover, GSPE treatment induced heparin-releasable lipoprotein lipase activity in the RWAT and MWAT depots. The alterations in the lipid metabolic pathways induced by GSPE were accompanied by lower FFA levels in the plasma and decreased lipid and triglyceride accumulation in the MWAT.
Conclusion:
The use of GSPE at low doses protects against fat accumulation and improves the plasma lipid profile in hamsters. We suggest that GSPE exerts these effects in part through the activation of both β-oxidation and the GL/FFA cycle, mainly in the RWAT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Galgani J, Ravussin E . Energy metabolism, fuel selection and body weight regulation. Int J Obes (Lond) 2008; 32: S109–S119.
Hubacek JA . Eat less and exercise more—is it really enough to knock down the obesity pandemia? Physiol Res/Acad Scient Bohemoslov 2009; 58 (Suppl 1) S1–S6.
Naukkarinen J, Rissanen A, Kaprio J, Pietilainen KH . Causes and consequences of obesity: the contribution of recent twin studies. Int J Obes (Lond) 2011. e-pub ahead of print 11 October 2011; doi:10.1038/ijo.2011.192.
Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX et al Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Arterioscler Thromb Vasc Biol 2006; 26: 968–976.
Azagury DE, Lautz DB . Obesity overview: epidemiology, health and financial impact, and guidelines for qualification for surgical therapy. Gastrointest Endosc Clin N Am 2011; 21: 189–201.
Bray GA . Are non-prescription medications needed for weight control? Obesity (Silver Spring, MD) 2008; 16: 509–514.
Pinent M, Blade C, Salvado MJ, Blay M, Pujadas G, Fernandez-Larrea J et al Procyanidin effects on adipocyte-related pathologies. Crit Rev Food Sci Nutr 2006; 46: 543–550.
Hsu CL, Yen GC . Phenolic compounds: evidence for inhibitory effects against obesity and their underlying molecular signaling mechanisms. Mol Nutr Food Res 2008; 52: 53–61.
Meydani M, Hasan ST . Dietary polyphenols and obesity. Nutrients 2010; 2: 737–751.
Blade C, Arola L, Salvado MJ . Hypolipidemic effects of proanthocyanidins and their underlying biochemical and molecular mechanisms. Mol Nutr Food Res 2010; 54: 37–59.
Rains TM, Agarwal S, Maki KC . Antiobesity effects of green tea catechins: a mechanistic review. J Nutr Biochem 2011; 22: 1–7.
Grove KA, Lambert JD . Laboratory, epidemiological, and human intervention studies show that tea (Camellia sinensis) may be useful in the prevention of obesity. J Nutr 2010; 140: 446–453.
Ahn J, Cho I, Kim S, Kwon D, Ha T . Dietary resveratrol alters lipid metabolism-related gene expression of mice on an atherogenic diet. J Hepatol 2008; 49: 1019–1028.
Macarulla MT, Alberdi G, Gomez S, Tueros I, Bald C, Rodriguez VM et al Effects of different doses of resveratrol on body fat and serum parameters in rats fed a hypercaloric diet. J Physiol Biochem 2009; 65: 369–376.
Rivera L, Moron R, Zarzuelo A, Galisteo M . Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem Pharmacol 2009; 77: 1053–1063.
Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F et al Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006; 127: 1109–1122.
Del Bas JM, Fernandez-Larrea J, Blay M, Ardevol A, Salvado MJ, Arola L et al Grape seed procyanidins improve atherosclerotic risk index and induce liver CYP7A1 and SHP expression in healthy rats. FASEB J 2005; 19: 479–481.
Del Bas JM, Ricketts ML, Baiges I, Quesada H, Ardevol A, Salvado MJ et al Dietary procyanidins lower triglyceride levels signaling through the nuclear receptor small heterodimer partner. Mol Nutr Food Res 2008; 52: 1172–1181.
Del Bas JM, Ricketts ML, Vaque M, Sala E, Quesada H, Ardevol A et al Dietary procyanidins enhance transcriptional activity of bile acid-activated FXR in vitro and reduce triglyceridemia in vivo in a FXR-dependent manner. Mol Nutr Food Res 2009; 53: 805–814.
Quesada H, del Bas JM, Pajuelo D, Diaz S, Fernandez-Larrea J, Pinent M et al Grape seed proanthocyanidins correct dyslipidemia associated with a high-fat diet in rats and repress genes controlling lipogenesis and VLDL assembling in liver. Int J Obes (Lond) 2009; 33: 1007–1012.
Pajuelo D, Diaz S, Quesada H, Fernandez-Iglesias A, Mulero M, Arola-Arnal A et al Acute administration of grape seed proanthocyanidin extract modulates energetic metabolism in skeletal muscle and BAT mitochondria. J Agric Food Chem 2011; 59: 4279–4287.
Auger C, Caporiccio B, Landrault N, Teissedre PL, Laurent C, Cros G et al Red wine phenolic compounds reduce plasma lipids and apolipoprotein B and prevent early aortic atherosclerosis in hypercholesterolemic golden Syrian hamsters (Mesocricetus auratus). J Nutr 2002; 132: 1207–1213.
Terra X, Montagut G, Bustos M, Llopiz N, Ardevol A, Blade C et al Grape-seed procyanidins prevent low-grade inflammation by modulating cytokine expression in rats fed a high-fat diet. J Nutr Biochem 2009; 20: 210–218.
Terra X, Pallares V, Ardevol A, Blade C, Fernandez-Larrea J, Pujadas G et al Modulatory effect of grape-seed procyanidins on local and systemic inflammation in diet-induced obesity rats. J Nutr Biochem 2011; 22: 380–387.
Pinent M, Blay M, Blade MC, Salvado MJ, Arola L, Ardevol A . Grape seed-derived procyanidins have an antihyperglycemic effect in streptozotocin-induced diabetic rats and insulinomimetic activity in insulin-sensitive cell lines. Endocrinology 2004; 145: 4985–4990.
Montagut G, Blade C, Blay M, Fernandez-Larrea J, Pujadas G, Salvado MJ et al Effects of a grapeseed procyanidin extract (GSPE) on insulin resistance. J Nutr Biochem 2010; 21: 961–967.
Montagut G, Onnockx S, Vaque M, Blade C, Blay M, Fernandez-Larrea J et al Oligomers of grape-seed procyanidin extract activate the insulin receptor and key targets of the insulin signaling pathway differently from insulin. J Nutr Biochem 2010; 21: 476–481.
Decorde K, Teissedre PL, Auger C, Cristol JP, Rouanet JM . Phenolics from purple grape, apple, purple grape juice and apple juice prevent early atherosclerosis induced by an atherogenic diet in hamsters. Mol Nutr Food Res 2008; 52: 400–407.
Charradi K, Sebai H, Elkahoui S, Ben Hassine F, Limam F, Aouani E . Grape seed extract alleviates high-fat diet-induced obesity and heart dysfunction by preventing cardiac siderosis. Cardiovasc Toxicol 2011; 11: 28–37.
Pajuelo D, Quesada H, Diaz S, Fernandez-Iglesias A, Arola-Arnal A, Blade C et al Chronic dietary supplementation of proanthocyanidins corrects the mitochondrial dysfunction of brown adipose tissue caused by diet-induced obesity in Wistar rats. Br J Nutr 2012; 107: 170–178.
Agouni A, Lagrue-Lak-Hal AH, Mostefai HA, Tesse A, Mulder P, Rouet P et al Red wine polyphenols prevent metabolic and cardiovascular alterations associated with obesity in Zucker fatty rats (Fa/Fa). PLoS One 2009; 4: e5557.
Saura-Calixto F, Serrano J, Goñi I . Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem 2007; 101: 492–501.
Serrano J, Puupponen-Pimiä R, Dauer A, Aura AM, Saura-Calixto F . Tannins: current knowledge of food sources, intake, bioavailability and biological effects. Mol Nutr Food Res 2009; 53: 310–329.
Fuchs RK, Allen MR, Condon KW, Reinwald S, Miller LM, McClenathan D et al Calculating clinically relevant drug doses to use in animal studies. Osteoporos Int 2008; 19: 1815–1817.
Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 2001; 25: 402–408.
Perseghin G, Caumo A, Caloni M, Testolin G, Luzi L . Incorporation of the fasting plasma FFA concentration into QUICKI improves its association with insulin sensitivity in nonobese individuals. J Clin Endocrinol Metab 2001; 86: 4776–4781.
Borai A, Livingstone C, Ferns GA . The biochemical assessment of insulin resistance. Ann Clin Biochem 2007; 44 (Part 4) 324–342.
Hara A, Radin NS . Lipid extraction of tissues with a low-toxicity solvent. Anal Biochem 1978; 90: 420–426.
Rodriguez-Sureda V, Peinado-Onsurbe J . A procedure for measuring triacylglyceride and cholesterol content using a small amount of tissue. Anal Biochem 2005; 343: 277–282.
Caimari A, Oliver P, Palou A . Adipose triglyceride lipase expression and fasting regulation are differently affected by cold exposure in adipose tissues of lean and obese Zucker rats. J Nutr Biochem 2011. e-pub ahead of print 21 September 2011.
Ohyama K, Furuta C, Nogusa Y, Nomura K, Miwa T, Suzuki K . Catechin-rich grape seed extract supplementation attenuates diet-induced obesity in C57BL/6J mice. Ann Nutr Metab 2011; 58: 250–258.
Bose M, Lambert JD, Ju J, Reuhl KR, Shapses SA, Yang CS . The major green tea polyphenol, (−)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J Nutr 2008; 138: 1677–1683.
Klaus S, Pultz S, Thone-Reineke C, Wolfram S . Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. Int J Obes (Lond) 2005; 29: 615–623.
Zheng G, Sayama K, Okubo T, Juneja LR, Oguni I . Anti-obesity effects of three major components of green tea, catechins, caffeine and theanine, in mice. In Vivo 2004; 18: 55–62.
Murase T, Nagasawa A, Suzuki J, Hase T, Tokimitsu I . Beneficial effects of tea catechins on diet-induced obesity: stimulation of lipid catabolism in the liver. Int J Obes Relat Metab Disord 2002; 26: 1459–1464.
Vadillo M, Ardevol A, Fernandez-Larrea J, Pujadas G, Blade C, Salvado MJ et al Moderate red-wine consumption partially prevents body weight gain in rats fed a hyperlipidic diet. J Nutr Biochem 2006; 17: 139–142.
Tebib K, Besancon P, Rouanet JM . Dietary grape seed tannins affect lipoproteins, lipoprotein lipases and tissue lipids in rats fed hypercholesterolemic diets. J Nutr 1994; 124: 2451–2457.
Ikeda I, Tsuda K, Suzuki Y, Kobayashi M, Unno T, Tomoyori H et al Tea catechins with a galloyl moiety suppress postprandial hypertriacylglycerolemia by delaying lymphatic transport of dietary fat in rats. J Nutr 2005; 135: 155–159.
Ngamukote S, Makynen K, Thilawech T, Adisakwattana S . Cholesterol-lowering activity of the major polyphenols in grape seed. Molecules 2011; 16: 5054–5061.
Baiges I, Palmfeldt J, Blade C, Gregersen N, Arola L . Lipogenesis is decreased by grape seed proanthocyanidins according to liver proteomics of rats fed a high fat diet. Mol Cell Proteomics: MCP 2010; 9: 1499–1513.
Ardevol A, Blade C, Salvado MJ, Arola L . Changes in lipolysis and hormone-sensitive lipase expression caused by procyanidins in 3T3-L1 adipocytes. Int J Obes Relat Metab Disord 2000; 24: 319–324.
Pinent M, Blade MC, Salvado MJ, Arola L, Ardevol A . Intracellular mediators of procyanidin-induced lipolysis in 3T3-L1 adipocytes. J Agric Food Chem 2005; 53: 262–266.
Pinent M, Blade MC, Salvado MJ, Arola L, Ardevol A . Metabolic fate of glucose on 3T3-L1 adipocytes treated with grape seed-derived procyanidin extract (GSPE). Comparison with the effects of insulin. J Agric Food Chem 2005; 53: 5932–5935.
Prentki M, Madiraju SR . Glycerolipid metabolism and signaling in health and disease. Endocr Rev 2008; 29: 647–676.
Tordjman J, Chauvet G, Quette J, Beale EG, Forest C, Antoine B . Thiazolidinediones block fatty acid release by inducing glyceroneogenesis in fat cells. J Biol Chem 2003; 278: 18785–18790.
Zechner R, Strauss J, Frank S, Wagner E, Hofmann W, Kratky D et al The role of lipoprotein lipase in adipose tissue development and metabolism. Int J Obes Relat Metab Disord 2000; 24 (Suppl 4) S53–S56.
Oliver P, Ribot J, Rodriguez AM, Sanchez J, Pico C, Palou A . Resistin as a putative modulator of insulin action in the daily feeding/fasting rhythm. Pflugers Arch 2006; 1–8.
Caimari A, Oliver P, Palou A . Regulation of adiponutrin expression by feeding conditions in rats is altered in the obese state. Obesity (Silver Spring, MD) 2007; 15: 591–599.
Caimari A, Oliver P, Palou A . Impairment of nutritional regulation of adipose triglyceride lipase expression with age. Int J Obes (Lond) 2008; 32: 1193–1200.
Acknowledgements
The research leading to these results has received funding from ACC1Ó (TECCT11-1-0012) and from the European Union’s Seventh Framework Programme FP7 2007–2013 under Grant agreement no. 244995 (BIOCLAIMS Project). We gratefully acknowledge the aid of Mr David Moriñas for the statistical support, and also Dr Isabel Baiges, Dr Anna Arola and Vanessa Grifoll, the laboratory technician.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Caimari, A., del Bas, J., Crescenti, A. et al. Low doses of grape seed procyanidins reduce adiposity and improve the plasma lipid profile in hamsters. Int J Obes 37, 576–583 (2013). https://doi.org/10.1038/ijo.2012.75
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2012.75
Keywords
This article is cited by
-
Developing a model to predict the early risk of hypertriglyceridemia based on inhibiting lipoprotein lipase (LPL): a translational study
Scientific Reports (2023)
-
Physico-chemical Characterization and Biosafety Evaluation of Atorvastatin Nanocapsules Co-encapsulated with Ginger Oil or Grape Seed Oil
BioNanoScience (2023)
-
Time-of-day dependent effect of proanthocyanidins on adipose tissue metabolism in rats with diet-induced obesity
International Journal of Obesity (2022)
-
Grape seed extract: having a potential health benefits
Journal of Food Science and Technology (2020)
-
Alterations in gut microbiota associated with a cafeteria diet and the physiological consequences in the host
International Journal of Obesity (2018)