Abstract
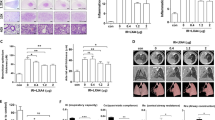
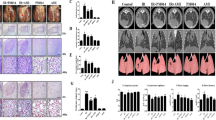
Forkhead transcription factor 3 (Foxp3) has a critical role in regulatory T cells (Treg). There are an increasing number of researches concerning the functions of Foxp3 in other cells, including lung epithelial cells besides Treg. However, the roles of Foxp3 in lung epithelial cells remain poorly understood. To examine the potential therapeutic benefits of Foxp3 for lung inflammation, this study investigates the effect of adenovirus-mediated Foxp3 overexpression in a radiation-induced lung damage model. Foxp3-EGFP expressing adenovirus was administered by intratracheal injection three times over 14 days after focal X-ray irradiation. To evaluate effects of Foxp3 overexpression in radiation-induced lung inflammation, immune cell profiles of bronchoalveolar lavage (BAL) fluid were analyzed. Foxp3 gene-delivered mice showed significant inhibition of immune cell infiltration, such as eosinophils, lymphocytes, macrophages and neutrophils in BAL fluid. Histopathological analysis also showed that Foxp3 overexpression inhibits inflammatory cell recruitment and collagen deposition in lung tissues. In addition, expression of inflammatory and fibrosis-related genes was decreased in the Foxp3 expression adenovirus-infected group. These results suggest that Foxp3 expression in lungs holds considerable therapeutic potential for attenuating inflammation and fibrosis in radiation-induced lung injury.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Chung HS, Lee JH, Kim H, Lee HJ, Kim SH, Kwon HK et al. Foxp3 is a novel repressor of microglia activation. Glia 2010; 58: 1247–1256.
Devaud C, Yong CS, John LB, Westwood JA, Duong CP, House CM et al. Foxp3 expression in macrophages associated with RENCA tumors in mice. PLoS One 2014; 9: e108670.
Chen GY, Chen C, Wang L, Chang X, Zheng P, Liu Y . Cutting edge: broad expression of the FoxP3 locus in epithelial cells: a caution against early interpretation of fatal inflammatory diseases following in vivo depletion of FoxP3-expressing cells. J Immunol 2008; 180: 5163–5166.
Sohn SH, Ko E, Oh BG, Kim SH, Kim Y, Shin M et al. Inhibition effects of Vitex rotundifolia on inflammatory gene expression in A549 human epithelial cells. Ann Allergy Asthma Immunol 2009; 103: 152–159.
McDonald S, Rubin P, Phillips TL, Marks LB . Injury to the lung from cancer therapy: clinical syndromes, measurable endpoints, and potential scoring systems. Int J Radiat Oncol Biol Phys 1995; 31: 1187–1203.
Ding NH, Li JJ, Sun LQ . Molecular mechanisms and treatment of radiation-induced lung fibrosis. Curr Drug Targets 2013; 14: 1347–1356.
Benveniste MF, Welsh J, Godoy MC, Betancourt SL, Mawlawi OR, Munden RF . New era of radiotherapy: an update in radiation-induced lung disease. Clin Radiol 68: e275–e290.
Almeida C, Nagarajan D, Tian J, Leal SW, Wheeler K, Munley M et al. The role of alveolar epithelium in radiation-induced lung injury. PLoS One 8: e53628.
Begg AC, Stewart FA, Vens C . Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer 2011; 11: 239–253.
Angel E, Yaghmai N, Jude CM, DeMarco JJ, Cagnon CH, Goldin JG et al. Dose to radiosensitive organs during routine chest CT: effects of tube current modulation. AJR Am J Roentgenol 2009; 193: 1340–1345.
Wu Z, Wang X, Yang R, Liu Y, Zhao W, Si J et al. Effects of carbon ion beam irradiation on lung injury and pulmonary fibrosis in mice. Exp Ther Med 2013; 5: 771–776.
Vorburger SA, Hunt KK . Adenoviral gene therapy. Oncologist 2002; 7: 46–59.
Epperly MW, Bray JA, Krager S, Berry LM, Gooding W, Engelhardt JF et al. Intratracheal injection of adenovirus containing the human MnSOD transgene protects athymic nude mice from irradiation-induced organizing alveolitis. Int J Radiat Oncol Biol Phys 1999; 43: 169–181.
Anscher MS, Murase T, Prescott DM, Marks LB, Reisenbichler H, Bentel GC et al. Changes in plasma TGF beta levels during pulmonary radiotherapy as a predictor of the risk of developing radiation pneumonitis. Int J Radiat Oncol Biol Phys 1994; 30: 671–676.
Hiemstra PS, McCray Jr PB, Bals R . The innate immune function of airway epithelial cells in inflammatory lung disease. Eur Respir J 2015; 45: 1150–1162.
Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN . House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med 2009; 15: 410–416.
Vigne S, Palmer G, Lamacchia C, Martin P, Talabot-Ayer D, Rodriguez E et al. IL-36 R ligands are potent regulators of dendritic and T cells. Blood 2011; 118: 5813–5823.
Zhang M, Qian YY, Chai SJ, Liang ZY, Xu Q, Wu ZQ et al. Enhanced local Foxp3 expression in lung tissue attenuates airway inflammation in a mouse model of asthma. J Asthma 2014; 51: 451–458.
Mays LE, Ammon-Treiber S, Mothes B, Alkhaled M, Rottenberger J, Muller-Hermelink ES et al. Modified Foxp3 mRNA protects against asthma through an IL-10-dependent mechanism. J Clin Invest 2013; 123: 1216–1228.
Wang JY, Chen KY, Wang JT, Chen JH, Lin JW, Wang HC et al. Outcome and prognostic factors for patients with non-small-cell lung cancer and severe radiation pneumonitis. Int J Radiat Oncol Biol Phys 2002; 54: 735–741.
Robnett TJ, Machtay M, Vines EF, McKenna MG, Algazy KM, McKenna WG . Factors predicting severe radiation pneumonitis in patients receiving definitive chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys 2000; 48: 89–94.
Ghafoori P, Marks LB, Vujaskovic Z, Kelsey CR . Radiation-induced lung injury. Assessment, management, and prevention. Oncology (Williston Park) 2008; 22: 37–47; discussion 52–53.
Lee JC, Kinniry PA, Arguiri E, Serota M, Kanterakis S, Chatterjee S et al. Dietary curcumin increases antioxidant defenses in lung, ameliorates radiation-induced pulmonary fibrosis, and improves survival in mice. Radiat Res 2010; 173: 590–601.
Trovo M, Linda A, El Naqa I, Javidan-Nejad C, Bradley J . Early and late lung radiographic injury following stereotactic body radiation therapy (SBRT). Lung Cancer 2010; 69: 77–85.
Schefter TE, Kavanagh BD, Timmerman RD, Cardenes HR, Baron A, Gaspar LE . A phase I trial of stereotactic body radiation therapy (SBRT) for liver metastases. Int J Radiat Oncol Biol Phys 2005; 62: 1371–1378.
Uematsu M, Shioda A, Suda A, Fukui T, Ozeki Y, Hama Y et al. Computed tomography-guided frameless stereotactic radiotherapy for stage I non-small cell lung cancer: a 5-year experience. Int J Radiat Oncol Biol Phys 2001; 51: 666–670.
Nagata Y, Takayama K, Matsuo Y, Norihisa Y, Mizowaki T, Sakamoto T et al. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys 2005; 63: 1427–1431.
Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 2003; 300: 1155–1159.
Fuks Z, Kolesnick R . Engaging the vascular component of the tumor response. Cancer Cell 2005; 8: 89–91.
Hong ZY, Eun SH, Park K, Choi WH, Lee JI, Lee EJ et al. Development of a small animal model to simulate clinical stereotactic body radiotherapy-induced central and peripheral lung injuries. J Radiat Res 2014; 55: 648–657.
Zhang H, Han G, Liu H, Chen J, Ji X, Zhou F et al. The development of classically and alternatively activated macrophages has different effects on the varied stages of radiation-induced pulmonary injury in mice. J Radiat Res 2011; 52: 717–726.
Borthwick LA, Wynn TA, Fisher AJ . Cytokine mediated tissue fibrosis. Biochim Biophys Acta 2013; 1832: 1049–1060.
Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1). J Exp Med 2001; 194: 809–821.
Graves PR, Siddiqui F, Anscher MS, Movsas B . Radiation pulmonary toxicity: from mechanisms to management. Semin Radiat Oncol 2010; 20: 201–207.
Saito F, Tasaka S, Inoue K, Miyamoto K, Nakano Y, Ogawa Y et al. Role of interleukin-6 in bleomycin-induced lung inflammatory changes in mice. Am J Respir Cell Mol Biol 2008; 38: 566–571.
Shihab FS, Yamamoto T, Nast CC, Cohen AH, Noble NA, Gold LI et al. Transforming growth factor-beta and matrix protein expression in acute and chronic rejection of human renal allografts. J Am Soc Nephrol 1995; 6: 286–294.
Puthawala K, Hadjiangelis N, Jacoby SC, Bayongan E, Zhao Z, Yang Z et al. Inhibition of integrin alpha(v)beta6, an activator of latent transforming growth factor-beta, prevents radiation-induced lung fibrosis. Am J Respir Crit Care Med 2008; 177: 82–90.
Branton MH, Kopp JB . TGF-beta and fibrosis. Microbes Infect 1999; 1: 1349–1365.
Martin M, Lefaix J, Delanian S . TGF-beta1 and radiation fibrosis: a master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys 2000; 47: 277–290.
Leask A, Abraham DJ . TGF-beta signaling and the fibrotic response. FASEB J 2004; 18: 816–827.
Wahl SM, Hunt DA, Wakefield LM, McCartney-Francis N, Wahl LM, Roberts AB et al. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci USA 1987; 84: 5788–5792.
Johnston CJ, Williams JP, Okunieff P, Finkelstein JN . Radiation-induced pulmonary fibrosis: examination of chemokine and chemokine receptor families. Radiat Res 2002; 157: 256–265.
Sohn SH, Lee JM, Park S, Yoo H, Kang JW, Shin D et al. The inflammasome accelerates radiation-induced lung inflammation and fibrosis in mice. Env Toxicol Pharmacol 2015; 39: 917–926.
Romero-Vargas FF, Ponce-Soto LA, Martins-de-Souza D, Marangoni S . Biological and biochemical characterization of two new PLA2 isoforms Cdc-9 and Cdc-10 from Crotalus durissus cumanensis snake venom. Comp Biochem Physiol C Toxicol Pharmacol 151: 66–74.
Ashcroft T, Simpson JM, Timbrell V . Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol 1988; 41: 467–470.
Acknowledgements
This work was supported by the Convergence of Conventional Medicine and Traditional Korean Medicine R&D program (JC and HB, HI15C0214) and the Nuclear Research and Development Program (JC, NRF-2011-0031695).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Shin, D., Lee, G., Lee, S. et al. Adenovirus-mediated Foxp3 expression in lung epithelial cells ameliorates acute radiation-induced pneumonitis in mice. Gene Ther 24, 104–112 (2017). https://doi.org/10.1038/gt.2016.86
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2016.86
This article is cited by
-
Pyroptotic cell death: an emerging therapeutic opportunity for radiotherapy
Cell Death Discovery (2024)