Abstract
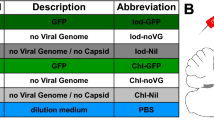
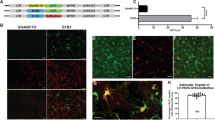
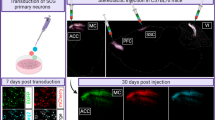
Adeno-associated virus (AAV) vectors are showing promise in gene therapy trials and have proven to be extremely efficient biological tools in basic neuroscience research. One major limitation to their widespread use in the neuroscience laboratory is the cost, labor, skill and time-intense purification process of AAV. We have recently shown that AAV can associate with exosomes (exo-AAV) when the vector is isolated from conditioned media of producer cells, and the exo-AAV is more resistant to neutralizing anti-AAV antibodies compared with standard AAV. Here, we demonstrate that simple pelleting of exo-AAV from media via ultracentrifugation results in high-titer vector preparations capable of efficient transduction of central nervous system (CNS) cells after systemic injection in mice. We observed that exo-AAV is more efficient at gene delivery to the brain at low vector doses relative to conventional AAV, even when derived from a serotype that does not normally efficiently cross the blood–brain barrier. Similar cell types were transduced by exo-AAV and conventionally purified vector. Importantly, no cellular toxicity was noted in exo-AAV-transduced cells. We demonstrated the utility and robustness of exo-AAV-mediated gene delivery by detecting direct GFP fluorescence after systemic injection, allowing three-dimensional reconstruction of transduced Purkinje cells in the cerebellum using ex vivo serial two-photon tomography. The ease of isolation combined with the high efficiency of transgene expression in the CNS, may enable the widespread use of exo-AAV as a neuroscience research tool. Furthermore, the ability of exo-AAV to evade neutralizing antibodies while still transducing CNS after peripheral delivery is clinically relevant.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Kalia LV, Kalia SK, Lang AE . Disease-modifying strategies for Parkinson's disease. Mov Disord 2015; 30: 1442–1450.
Muramatsu S . Gene therapy for Parkinson disease. Nihon Rinsho 2010; 68: 646–649.
Bennett J, Ashtari M, Wellman J, Marshall KA, Cyckowski LL, Chung DC et al. AAV2 gene therapy readministration in three adults with congenital blindness. Sci Transl Med 2012; 4: 120ra15.
Maguire AM, High KA, Auricchio A, Wright JF, Pierce EA, Testa F et al. Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a phase 1 dose-escalation trial. Lancet 2009; 374: 1597–1605.
Worgall S, Sondhi D, Hackett NR, Kosofsky B, Kekatpure MV, Neyzi N et al. Treatment of late infantile neuronal ceroid lipofuscinosis by CNS administration of a serotype 2 adeno-associated virus expressing CLN2 cDNA. Hum Gene Ther 2008; 19: 463–474.
Janson C, McPhee S, Bilaniuk L, Haselgrove J, Testaiuti M, Freese A et al. Clinical protocol. Gene therapy of Canavan disease: AAV-2 vector for neurosurgical delivery of aspartoacylase gene (ASPA) to the human brain. Hum Gene Ther 2002; 13: 1391–1412.
Yang B, Li S, Wang H, Guo Y, Gessler DJ, Cao C et al. Global CNS transduction of adult mice by intravenously delivered rAAVrh.8 and rAAVrh.10 and nonhuman primates by rAAVrh.10. Mol Ther 2014; 22: 1299–1309.
Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK . Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol 2009; 27: 59–65.
Gaj T, Epstein BE, Schaffer DV . Genome engineering using adeno-associated virus: basic and clinical research applications. Mol Ther e-pub ahead of print 16 September 2015.
Swiech L, Heidenreich M, Banerjee A, Habib N, Li Y, Trombetta J et al. In vivo interrogation of gene function in the mammalian brain using CRISPR-Cas9. Nat Biotechnol 2015; 33: 102–106.
Tang W, Szokol K, Jensen V, Enger R, Trivedi CA, Hvalby O et al. Stimulation-evoked Ca2+ signals in astrocytic processes at hippocampal CA3-CA1 synapses of adult mice are modulated by glutamate and ATP. J Neurosci 2015; 35: 3016–3021.
Ekstrand MI, Nectow AR, Knight ZA, Latcha KN, Pomeranz LE, Friedman JM . Molecular profiling of neurons based on connectivity. Cell 2014; 157: 1230–1242.
Xu W, Sudhof TC . A neural circuit for memory specificity and generalization. Science 2013; 339: 1290–1295.
Cai D, Cohen KB, Luo T, Lichtman JW, Sanes JR . Improved tools for the Brainbow toolbox. Nat Methods 2013; 10: 540–547.
Shimano T, Fyk-Kolodziej B, Mirza N, Asako M, Tomoda K, Bledsoe S et al. Assessment of the AAV-mediated expression of channelrhodopsin-2 and halorhodopsin in brainstem neurons mediating auditory signaling. Brain Res 2013; 1511: 138–152.
Kaspar BK, Vissel B, Bengoechea T, Crone S, Randolph-Moore L, Muller R et al. Adeno-associated virus effectively mediates conditional gene modification in the brain. Proc Natl Acad Sci USA 2002; 99: 2320–2325.
Maguire CA, Balaj L, Sivaraman S, Crommentuijn MH, Ericsson M, Mincheva-Nilsson L et al. Microvesicle-associated AAV vector as a novel gene delivery system. Mol Ther 2012; 20: 960–971.
Gyorgy B, Fitzpatrick Z, Crommentuijn MH, Mu D, Maguire CA . Naturally enveloped AAV vectors for shielding neutralizing antibodies and robust gene delivery in vivo. Biomaterials 2014; 35: 7598–7609.
Balaj L, Atai NA, Chen W, Mu D, Tannous BA, Breakefield XO et al. Heparin affinity purification of extracellular vesicles. Sci Rep 2015; 5: 10266.
Hawkins BT, Grego S, Sellgren KL . Three-dimensional culture conditions differentially affect astrocyte modulation of brain endothelial barrier function in response to transforming growth factor beta1. Brain Res 2015; 1608: 167–176.
Kotin RM . Large-scale recombinant adeno-associated virus production. Hum Mol Genet 2011; 20: R2–R6.
Zhang H, Xie J, Xie Q, Wilson JM, Gao G . Adenovirus-adeno-associated virus hybrid for large-scale recombinant adeno-associated virus production. Hum Gene Ther 2009; 20: 922–929.
de Rivero Vaccari JP, Brand F 3rd, Adamczak S, Lee SW, Barcena JP, Wang MY et al. Exosome-mediated inflammasome signaling after central nervous system injury. J Neurochem 2015.
Rajendran L, Bali J, Barr MM, Court FA, Kramer-Albers EM, Picou F et al. Emerging roles of extracellular vesicles in the nervous system. J Neurosci 2014; 34: 15482–15489.
Pegtel DM, Peferoen L, Amor S . Extracellular vesicles as modulators of cell-to-cell communication in the healthy and diseased brain. Philos Trans R Soc Lond B Biol Sci 2014; 369: pii: 20130516.
Volterra A, Liaudet N, Savtchouk I . Astrocyte Ca(2)(+) signalling: an unexpected complexity. Nat Rev Neurosci 2014; 15: 327–335.
Ragan T, Kadiri LR, Venkataraju KU, Bahlmann K, Sutin J, Taranda J et al. Serial two-photon tomography for automated ex vivo mouse brain imaging. Nat Methods 2012; 9: 255–258.
Bemelmans AP, Duque S, Riviere C, Astord S, Desrosiers M, Marais T et al. A single intravenous AAV9 injection mediates bilateral gene transfer to the adult mouse retina. PLoS One 2013; 8: e61618.
Luebke AE, Rova C, Von Doersten PG, Poulsen DJ . Adenoviral and AAV-mediated gene transfer to the inner ear: role of serotype, promoter, and viral load on in vivo and in vitro infection efficiencies. Adv Otorhinolaryngol 2009; 66: 87–98.
Wood MJ, O'Loughlin AJ, Samira L . Exosomes and the blood-brain barrier: implications for neurological diseases. Ther Deliv 2011; 2: 1095–1099.
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ . Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 2011; 29: 341–345.
Lai CP, Mardini O, Ericsson M, Prabhakar S, Maguire CA, Chen JW et al. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano 2014; 8: 483–494.
Hareendran S, Balakrishnan B, Sen D, Kumar S, Srivastava A, Jayandharan GR . Adeno-associated virus (AAV) vectors in gene therapy: immune challenges and strategies to circumvent them. Rev Med Virol 2013; 23: 399–413.
Rogers GL, Martino AT, Aslanidi GV, Jayandharan GR, Srivastava A, Herzog RW . Innate Immune Responses to AAV Vectors. Front Microbiol 2011; 2: 194.
Gray SJ, Matagne V, Bachaboina L, Yadav S, Ojeda SR, Samulski RJ . Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Mol Ther 2011; 19: 1058–1069.
Maguire CA, Crommentuijn MH, Mu D, Hudry E, Serrano-Pozo A, Hyman BT et al. Mouse gender influences brain transduction by intravascularly administered AAV9. Mol Ther 2013; 21: 1470–1471.
Hudry E, Van Dam D, Kulik W, De Deyn PP, Stet FS, Ahouansou O et al. Adeno-associated virus gene therapy with cholesterol 24-hydroxylase reduces the amyloid pathology before or after the onset of amyloid plaques in mouse models of Alzheimer's disease. Mol Ther 2010; 18: 44–53.
Hudry E, Dashkoff J, Roe AD, Takeda S, Koffie RM, Hashimoto T et al. Gene transfer of human Apoe isoforms results in differential modulation of amyloid deposition and neurotoxicity in mouse brain. Sci Transl Med 2013; 5: 212ra161.
Spires TL, Meyer-Luehmann M, Stern EA, McLean PJ, Skoch J, Nguyen PT et al. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J Neurosci 2005; 25: 7278–7287.
Serrano-Pozo A, Muzikansky A, Gomez-Isla T, Growdon JH, Betensky RA, Frosch MP et al. Differential relationships of reactive astrocytes and microglia to fibrillar amyloid deposits in Alzheimer disease. J Neuropathol Exp Neurol 2013; 72: 462–471.
Persidsky Y, Fan S, Dykstra H, Reichenbach NL, Rom S, Ramirez SH . Activation of Cannabinoid Type Two Receptors (CB2) Diminish Inflammatory Responses in Macrophages and Brain Endothelium. J Neuroimmune Pharmacol 2015; 10: 302–308.
Acknowledgements
We thank Dr Miguel Sena-Esteves for providing the AAV plasmids as well as Dr David P. Corey for the expertise and resources of his laboratory on the cochlear transduction analysis. This work was supported by NIH/NINDS R21 NS081374-01 (CM), an American Brain Tumor Association Discovery grant (CM) and the Alzheimer’s Drug Discovery Foundation (EH) and JPB Foundation (BTH). In addition, this work was also partially supported by NIH/NINDS R01 NS086570-01 (SHR). We would like to acknowledge the Nucleic Acid Quantitation Core at MGH Neuroscience Center for qPCR analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
CM has a patent application related to the Exo-AAV (vexosome) technology and has received royalty payments from agreements between Partners Healthcare and Exosome Diagnostics, Inc. CM has received an honorarium from Biogen Idec for giving a seminar on the technology. The other authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Rights and permissions
About this article
Cite this article
Hudry, E., Martin, C., Gandhi, S. et al. Exosome-associated AAV vector as a robust and convenient neuroscience tool. Gene Ther 23, 380–392 (2016). https://doi.org/10.1038/gt.2016.11
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2016.11
This article is cited by
-
Distributional comparison of different AAV vectors after unilateral cochlear administration
Gene Therapy (2024)
-
Therapeutic management of ischemic stroke
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)
-
The encephalomyocarditis virus Leader promotes the release of virions inside extracellular vesicles via the induction of secretory autophagy
Nature Communications (2022)
-
Targeted delivery of therapeutic agents to the heart
Nature Reviews Cardiology (2021)
-
Syntenin-knock out reduces exosome turnover and viral transduction
Scientific Reports (2021)