Abstract
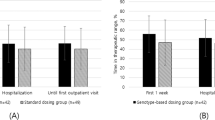
In terms of inconsistent conclusions across all relevant randomized controlled trials (RCTs) and available meta-analyses, we aimed to use a meta-analysis and trial sequential analysis (TSA) to evaluate whether clinical utility of a genotype-guided warfarin initiation dosing algorithm could be better than that of a standard therapy regimen, and whether currently relevant evidence could be reliable and conclusive. Overall, 11 eligible RCTs involving 2677 patients were included for further analyses. Compared with fixed dose or clinically adjusted warfarin initiation dosing regimens, genotype-guided algorithms significantly increased time in therapeutic range, shortened time to first therapeutic international normalized ratio (INR) and time to stable doses, but did not show any marked improvements in excessive anticoagulation, bleeding events, thromboembolism, or all-cause mortality. Subgroup analyses revealed that, genotype-guided algorithms showed better control in the outcomes of time in therapeutic range or excessive anticoagulation than fixed-dose regimens rather than clinically adjusted regimens. Except for excessive anticoagulation, currently available evidence of all other outcomes was unreliable and inconclusive as determined with TSA. Our findings suggest that genotype-guided warfarin initiation dosing algorithms have superiority in the improvement of surrogate quality markers for anticoagulation control, but that this does not translate into statistically significant differences in clinical outcomes, which is largely because of the insufficient sample size in the RCTs analyzed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Jonas DE, Bryant Shilliday B, Laundon WR, Pignone M . Patient time requirements for anticoagulation therapy with warfarin. Med Decis Making 2010; 30: 206–216.
Kirley K, Qato DM, Kornfield R, Stafford RS, Alexander GC . National trends in oral anticoagulant use in the United States, 2007 to 2011. Circ Cardiovasc Qual Outcomes 2012; 5: 615–621.
Wysowski DK, Nourjah P, Swartz L . Bleeding complications with warfarin use: a prevalent adverse effect resulting in regulatory action. Arch Intern Med 2007; 167: 1414–1419.
Budnitz DS, Lovegrove MC, Shehab N, Richards CL . Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med 2011; 365: 2002–2012.
Voora D, McLeod HL, Eby C, Gage BF . The pharmacogenetics of coumarin therapy. Pharmacogenomics 2005; 6: 503–513.
Cooper GM, Johnson JA, Langaee TY, Feng H, Stanaway IB, Schwarz UI et al. A genome-wide scan for common genetic variants with a large influence on warfarin maintenance dose. Blood 2008; 112: 1022–1027.
Ferder NS, Eby CS, Deych E, Harris JK, Ridker PM, Milligan PE et al. Ability of VKORC1 and CYP2C9 to predict therapeutic warfarin dose during the initial weeks of therapy. J Thromb Haemost 2010; 8: 95–100.
Hillman MA, Wilke RA, Yale SH, Vidaillet HJ, Caldwell MD, Glurich I et al. A prospective, randomized pilot trial of model-based warfarin dose initiation using CYP2C9 genotype and clinical data. Clin Med Res 2005; 3: 137–145.
Anderson JL, Horne BD, Stevens SM, Grove AS, Barton S, Nicholas ZP et al. Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation. Circulation 2007; 116: 2563–2570.
Caraco Y, Blotnick S, Muszkat M . CYP2C9 genotype-guided warfarin prescribing enhances the efficacy and safety of anticoagulation: a prospective randomized controlled study. Clin Pharmacol Ther 2008; 83: 460–470.
Huang SW, Chen HS, Wang XQ, Huang L, Xu DL, Hu XJ et al. Validation of VKORC1 and CYP2C9 genotypes on interindividual warfarin maintenance dose: a prospective study in Chinese patients. Pharmacogenet Genomics 2009; 19: 226–234.
Burmester JK, Berg RL, Yale SH, Rottscheit CM, Glurich IE, Schmelzer JR et al. A randomized controlled trial of genotype-based Coumadin initiation. Genet Med 2011; 13: 509–518.
Borgman MP, Pendleton RC, McMillin GA, Reynolds KK, Vazquez S, Freeman A et al. Prospective pilot trial of PerMIT versus standard anticoagulation service management of patients initiating oral anticoagulation. Thromb Haemost 2012; 108: 561–569.
Wang M, Lang X, Cui S, Fei K, Zou L, Cao J et al. Clinical application of pharmacogenetic-based warfarin-dosing algorithm in patients of Han nationality after rheumatic valve replacement: a randomized and controlled trial. Int J Med Sci 2012; 9: 472–479.
Jonas DE, Evans JP, McLeod HL, Brode S, Lange LA, Young ML et al. Impact of genotype-guided dosing on anticoagulation visits for adults starting warfarin: a randomized controlled trial. Pharmacogenomics 2013; 14: 1593–1603.
Li J, Liu S, Yang JH, Guo W, Wang ZZ, Chen Y et al. A randomized controlled study of the VKORCl and CYP2C9 genotypes in guiding warfhrin therapy for pulmonary thromboembolism. Chin J Tuberc Respir Dis 2013; 36: 950–953.
Tang HL, Li XG, Zhang T, Xie HG, Zhai SD . Pharmacogenetics Algorithms for IndividualizingWarfarin Dosing: Meta-Analysis of Randomized Controlled Trials. Ther Drug Monit 2013; 35: 724–725, (abstract P2616).
Kimmel SE, French B, Kasner SE, Johnson JA, Anderson JL, Gage BF et al. A pharmacogenetic versus a clinical algorithm for warfarin dosing. N Engl J Med 2013; 369: 2283–2293.
Pirmohamed M, Burnside G, Eriksson N, Jorgensen AL, Toh CH, Nicholson T et al. A randomized trial of genotype-guided dosing of warfarin. N Engl J Med 2013; 369: 2294–2303.
Roberts A . Anticoagulation therapy: genotype-guided anticoagulation therapy-the jury is still out. Nat Rev Cardiol 2014; 11: 1.
Cavallari LH, Nutescu EA . Warfarin pharmacogenetics: to genotype or not to genotype, that is the question. Clin Pharmacol Ther 2014; 96: 22–24.
Stergiopoulos K, Brown DL . Genotype-guided vs clinical dosing of warfarin and its analogues: meta-analysis of randomized clinical trials. JAMA Intern Med 2014; 174: 1330–1338.
Franchini M, Mengoli C, Cruciani M, Bonfanti C, Mannucci PM . Effects on bleeding complications of pharmacogenetic testing for initial dosing of vitamin K antagonists: a systematic review and meta-analysis. J Thromb Haemost 2014; 12: 1480–1487.
Tang Q, Zou H, Guo C, Liu Z . Outcomes of pharmacogenetics-guided dosing of warfarin: a systematic review and meta-analysis. Int J Cardiol 2014; 175: 587–591.
Liao Z, Feng S, Ling P, Zhang G . Meta-analysis of randomized controlled trials reveals an improved clinical outcome of using genotype plus clinical algorithm for warfarin dosing. J Thromb Thrombolysis 2014; 39: 228–234.
Goulding R, Dawes D, Price M, Wilkie S, Dawes M . Genotype-guided drug prescribing: a systematic review and meta-analysis of randomized control trials. Br J Clin Pharmacol 2014.
Wetterslev J, Thorlund K, Brok J, Gluud C . Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol 2008; 61: 64–75.
Moher D, Liberati A, Tetzlaff J, Altman DG . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med 2009; 3: e123–e130.
Higgins JPT, Green S . The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. 2011, Available from: www.cochrane.org.
Higgins JP, Thompson SG, Deeks JJ, Altman DG . Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560.
Yang J, Chen Y, Li X, Wei X, Chen X, Zhang L et al. Influence of CYP2C9 and VKORC1 genotypes on the risk of hemorrhagic complications in warfarin-treated patients: a systematic review and meta-analysis. Int J Cardiol 2013; 168: 4234–4243.
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J et al. GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011; 64: 383–394.
Gallagher AM, Setakis E, Plumb JM, Clemens A, van Staa TP . Risks of stroke and mortality associated with suboptimal anticoagulation in atrial fibrillation patients. Thromb Haemost 2011; 106: 968–977.
Hylek EM, Go AS, Chang Y, Jensvold NG, Henault LE, Selby JV et al. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med 2003; 349: 1019–1026.
Sidiropoulos N, Wu AH . Clinical trials for pharmacogenomics testing for warfarin dosing: relevance to general community practices. Genet Med 2011; 13: 505–508.
Kangelaris KN, Bent S, Nussbaum RL, Garcia DA, Tice JA . Genetic testing before anticoagulation? A systematic review of pharmacogenetic dosing of warfarin. J Gen Intern Med 2009; 24: 656–664.
Acknowledgements
This work was supported, in part, by a grant BL2013001 from the Department of Science and Technology of Jiangsu Province, and BK2012525 from the Natural Science Foundation of Jiangsu Province, China (both to HGX).
Author Contributions
HLT did the literature search, study selection, data extraction, statistical analysis, prepared tables and figures, and drafted the Methods and Results sections of the report. WLS did the literature search, study selection, and data extraction. XGL and TZ guided the statistical analysis, and interpreted the data. SDZ reviewed the data from gathered studies, and interpreted the data. HGX proposed the idea for this meta-analysis, interpreted the data, drafted Abstract, Introduction, Results and Discussion sections of the report, and finalized the whole manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Tang, H., Shi, W., Li, X. et al. Limited clinical utility of genotype-guided warfarin initiation dosing algorithms versus standard therapy: a meta-analysis and trial sequential analysis of 11 randomized controlled trials. Pharmacogenomics J 15, 496–504 (2015). https://doi.org/10.1038/tpj.2015.16
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tpj.2015.16
This article is cited by
-
Pharmacokinetic and pharmacodynamic re-evaluation of a genetic-guided warfarin trial
European Journal of Clinical Pharmacology (2018)
-
Assessing the relative potency of (S)- and (R)-warfarin with a new PK-PD model, in relation to VKORC1 genotypes
European Journal of Clinical Pharmacology (2017)