Abstract
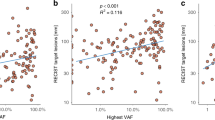
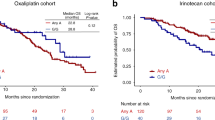
The number of CA tandem repeats (CA)n in a highly polymorphic region of EGFR (epidermal growth factor receptor) intron 1 may affect gene transcription; the potential impact of allelic variants on the efficacy of cetuximab in metastatic colorectal cancer (mCRC) patients is debated for long. This study aimed at prospectively estimating the impact of EGFR intron 1 (CA)n variants on clinical outcome in KRAS exon 2 and BRAF wild-type chemo-refractory mCRC patients, receiving cetuximab and irinotecan. Variants presenting
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009; 360: 1408–1417.
Bokemeyer C, Bondarenko I, Hartmann JT, de Braud F, Schuch G, Zubel A et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol 2011; 22: 1535–1546.
Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 2008; 26: 1626–1634.
Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008; 359: 1757–1765.
Loupakis F, Cremolini C, Fontanini G, Stasi I, Salvatore L, Falcone A . Beyond KRAS: perspectives on new potential markers of intrinsic and acquired resistance to epidermal growth factor receptor inhibitors in metastatic colorectal cancer. Ther Adv Med Oncol 2009; 1: 167–181.
De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol 2010; 11: 753–762.
Di Nicolantonio F, Martini M, Molinari F, Sartore-Bianchi A, Arena S, Saletti P et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol 2008; 26: 5705–5712.
Graziano F, Ruzzo A, Loupakis F, Canestrari E, Santini D, Catalano V et al. Pharmacogenetic profiling for cetuximab plus irinotecan therapy in patients with refractory advanced colorectal cancer. J Clin Oncol 2008; 26: 1427–1434.
Lurje G, Nagashima F, Zhang W, Yang D, Chang HM, Gordon MA et al. Polymorphisms in cyclooxygenase-2 and epidermal growth factor receptor are associated with progression-free survival independent of K-ras in metastatic colorectal cancer patients treated with single-agent cetuximab. Clin Cancer Res 2008; 14: 7884–7895.
Pander J, Gelderblom H, Antonini NF, Tol J, van Krieken JH, van der Straaten T et al. Correlation of FCGR3A and EGFR germline polymorphisms with the efficacy of cetuximab in KRAS wild-type metastatic colorectal cancer. Eur J Cancer 2010; 46: 1829–1834.
Park JH, Han SW, Oh DY, Im SA, Jeong SY, Park KJ et al. Analysis of KRAS, BRAF, PTEN, IGF1R, EGFR intron 1 CA status in both primary tumors and paired metastases in determining benefit from cetuximab therapy in colon cancer. Cancer Chemother Pharmacol 2011; 68: 1045–1055.
Dahan L, Norguet E, Etienne-Grimaldi MC, Formento JL, Gasmi M, Nanni I et al. Pharmacogenetic profiling and cetuximab outcome in patients with advanced colorectal cancer. BMC Cancer 2011; 11: 496.
Stintzing S, Kapaun C, Laubender RP, Jung A, Neumann J, Modest DP et al. Prognostic value of cetuximab-related skin toxicity in metastatic colorectal cancer patients and its correlation with parameters of the epidermal growth factor receptor signal transduction pathway: results from a randomized trial of the GERMAN AIO CRC Study Group. Int J Cancer 2013; 132: 236–245.
Lai CY, Sung FC, Hsieh LL, Tang R, Chiou HY, Wu FY et al. Associations between genetic polymorphisms of epidermal growth factor receptor (EGFR) and survival of colorectal cancer (CRC) patients treated with 5-fluorouracil-based chemotherapy. Ann Surg Oncol 2013; 20: 599–606.
Gebhardt F, Zanker KS, Brandt B . Modulation of epidermal growth factor receptor gene transcription by a polymorphic dinucleotide repeat in intron 1. J Biol Chem 1999; 274: 13176–13180.
Amador ML, Oppenheimer D, Perea S, Maitra A, Cusatis G, Iacobuzio-Donahue C et al. An epidermal growth factor receptor intron 1 polymorphism mediates response to epidermal growth factor receptor inhibitors. Cancer Res 2004; 64: 9139–9143.
Yuan Z, Shin J, Wilson A, Goel S, Ling YH, Ahmed N et al. An A13 repeat within the 3'-untranslated region of epidermal growth factor receptor (EGFR) is frequently mutated in microsatellite instability colon cancers and is associated with increased EGFR expression. Cancer Res 2009; 69: 7811–7818.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–216.
Martin-Martorell P, Rosello S, Rodriguez-Braun E, Chirivella I, Bosch A, Cervantes A . Biweekly cetuximab and irinotecan in advanced colorectal cancer patients progressing after at least one previous line of chemotherapy: results of a phase II single institution trial. Br J Cancer 2008; 99: 455–458.
Pfeiffer P, Nielsen D, Bjerregaard J, Qvortrup C, Yilmaz M, Jensen B . Biweekly cetuximab and irinotecan as third-line therapy in patients with advanced colorectal cancer after failure to irinotecan, oxaliplatin and 5-fluorouracil. Ann Oncol 2008; 19: 1141–1145.
Tabernero J, Ciardiello F, Rivera F, Rodriguez-Braun E, Ramos FJ, Martinelli E et al. Cetuximab administered once every second week to patients with metastatic colorectal cancer: a two-part pharmacokinetic/pharmacodynamic phase I dose-escalation study. Ann Oncol 2010; 21: 1537–1545.
Brodowicz T, Ciuleanu TE, Radosavljevic D, Shacham-Shmueli E, Vrbanec D, Plate S et al. FOLFOX4 plus cetuximab administered weekly or every second week in the first-line treatment of patients with KRAS wild-type metastatic colorectal cancer: a randomized phase II CECOG study. Ann Oncol 2013; 24: 1769–1777.
Cremolini C, Loupakis F, Antoniotti C, Fioravanti A, Orlandi P, Salvatore L Prospective analysis of the early modulation of plasma amphiregulin (AR) during treatment with cetuximab and irinotecan in irinotecan-refractory metastatic colorectal cancer (mCRC) patients (pts). J Clin Oncol ASCO Annual Meeting Proceedings No 15_suppl (May 20 Supplement): (abstr 3602) 2013; 31.
Zhang W, Gordon M, Press OA, Rhodes K, Vallböhmer D, Yang DY et al. Cyclin D1 and epidermal growth factor polymorphisms associated with survival in patients with advanced colorectal cancer treated with Cetuximab. Pharmacogenet Genomics 2006; 16: 475–483.
Buyse M, Sargent DJ, Grothey A, Matheson A, de Gramont A . Biomarkers and surrogate end points—the challenge of statistical validation. Nat Rev Clin Oncol 2010; 7: 309–317.
Allegra CJ, Jessup JM, Somerfield MR, Hamilton SR, Hammond EH, Hayes DF et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol 2009; 27: 2091–2096.
Missiaglia E, Jacobs B, Di Narzo AF, Soneson C, Roth A, Bosman F Proximal and distal colon tumors as distinct biologic entities with different prognoses. J Clin Oncol ASCO Annual Meeting Proceedings No 15_suppl (May 20 Supplement): (abstr 3526) 2013; 31.
Buyse M, Michiels S, Sargent DJ, Grothey A, Matheson A, de Gramont A . Integrating biomarkers in clinical trials. Expert Rev Mol Diagn 2011; 11: 171–182.
Maitland ML, Ratain MJ, Cox NJ . Interpreting P values in pharmacogenetic studies: a call for process and perspective. J Clin Oncol 2007; 25: 4513–4515.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Loupakis, F., Antoniotti, C., Cremolini, C. et al. Prospective study of EGFR intron 1 (CA)n repeats variants as predictors of benefit from cetuximab and irinotecan in chemo-refractory metastatic colorectal cancer (mCRC) patients. Pharmacogenomics J 14, 322–327 (2014). https://doi.org/10.1038/tpj.2014.1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tpj.2014.1
Keywords
This article is cited by
-
Germline polymorphisms as biomarkers of tumor response in colorectal cancer patients treated with anti-EGFR monoclonal antibodies: a systematic review and meta-analysis
The Pharmacogenomics Journal (2017)
-
CA-SSR1 Polymorphism in Intron 1 of the EGFR Gene in Patients with Malignant Tumors Who Develop Acneiform Rash Associated with the Use of Cetuximab
Molecular Diagnosis & Therapy (2015)