Abstract
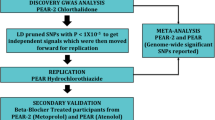
A recent genome-wide analysis discovered an association between a haplotype (from rs317689/rs315135/rs7297610) on Chromosome 12q15 and blood pressure response to hydrochlorothiazide (HCTZ) in African–Americans. Our aim was to replicate this association and investigate possible functional mechanisms. We observed similar associations between this haplotype and HCTZ response in an independent sample of 746 Caucasians and African–Americans randomized to HCTZ or atenolol treatment. The haplotype association was driven by variation at rs7297610, where C/C genotypes were associated with greater mean (systolic: 3.4 mmHg, P=0.0275; diastolic: 2.5 mmHg, P=0.0196) responses to HCTZ vs T-allele carriers. Such an association was absent in atenolol-treated participants, supporting this as HCTZ specific. Expression analyses in HCTZ-treated African–Americans showed differential pre-treatment leukocyte YEATS4 expression between rs7297610 genotype groups (P=0.024), and reduced post-treatment expression in C/C genotypes (P=0.009), but not in T-carriers. Our data confirm previous genome-wide findings at 12q15 and suggest differential YEATS4 expression could underpin rs7297610-associated HCTZ response variability, which may have future implications for guiding thiazide treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
2010 Top 200 generic drugs by total prescriptions. Drug Topics; http://drugtopics.modernmedicine.com/drugtopics/data/articlestandard//drugtopics/252011/727243/article.pdf; accessed on 19 July 2011.
2010 Top 200 branded drugs by total prescriptions. Drug Topics; http://drugtopics.modernmedicine.com/drugtopics/data/articlestandard//drugtopics/252011/727256/article.pdf; accessed on 19 July 2011.
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo Jr JL et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42: 1206–1252.
Duarte JD, Cooper-DeHoff RM . Mechanisms for blood pressure lowering and metabolic effects of thiazide and thiazide-like diuretics. Expert Rev Cardiovasc Ther 2010; 8: 793–802.
Carter BL, Einhorn PT, Brands M, He J, Cutler JA, Whelton PK et al. Thiazide-induced dysglycemia: call for research from a working group from the national heart, lung, and blood institute. Hypertension 2008; 52: 30–36.
Materson BJ . Variability in response to antihypertensive drugs. Am J Med 2007; 120 (4 Suppl 1): S10–S20.
Johnson JA, Gong Y, Bailey KR, Cooper-DeHoff RM, Chapman AB, Turner ST et al. Hydrochlorothiazide and atenolol combination antihypertensive therapy: effects of drug initiation order. Clin Pharmacol Ther 2009; 86: 533–539.
Comparison of propranolol and hydrochlorothiazide for the initial treatment of hypertension. II. Results of long-term therapy. [Veterans Administration Cooperative Study Group on Antihypertensive Agents.] JAMA 1982; 248: 2004–2011.
Turner ST, Bailey KR, Fridley BL, Chapman AB, Schwartz GL, Chai HS et al. Genomic association analysis suggests chromosome 12 locus influencing antihypertensive response to thiazide diuretic. Hypertension 2008; 52: 359–365.
Johnson JA, Boerwinkle E, Zineh I, Chapman AB, Bailey K, Cooper-DeHoff RM et al. Pharmacogenomics of antihypertensive drugs: rationale and design of the Pharmacogenomic Evaluation of Antihypertensive Responses (PEAR) study. Am Heart J 2009; 157: 442–449.
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007; 81: 559–575.
Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25: 402–408.
Chapman AB, Schwartz GL, Boerwinkle E, Turner ST . Predictors of antihypertensive response to a standard dose of hydrochlorothiazide for essential hypertension. Kidney Int 2002; 61: 1047–1055.
Zimmermann K, Ahrens K, Matthes S, Buerstedde JM, Stratling WH, Phi-van L . Targeted disruption of the GAS41 gene encoding a putative transcription factor indicates that GAS41 is essential for cell viability. J Biol Chem 2002; 277: 18626–18631.
Schulze JM, Wang AY, Kobor MS . YEATS domain proteins: a diverse family with many links to chromatin modification and transcription. Biochem cell biol 2009; 87: 65–75.
Park JH, Roeder RG . GAS41 is required for repression of the p53 tumor suppressor pathway during normal cellular proliferation. Mol cell biol 2006; 26: 4006–4016.
Abuladze N, Yanagawa N, Lee I, Jo OD, Newman D, Hwang J et al. Peripheral blood mononuclear cells express mutated NCCT mRNA in Gitelman's syndrome: evidence for abnormal thiazide-sensitive NaCl cotransport. J Am Soc Nephrol 1998; 9: 819–826.
Abraham AS, Brooks BA, Grafstein Y, Barchilon E, Nubani N, Eylath U et al. Effects of hydrochlorothiazide, diltiazem and enalapril on mononuclear cell sodium and magnesium levels in systemic hypertension. Am j cardiol 1991; 68: 1357–1361.
Acknowledgements
This work was supported by a Grant from the National Institutes of Health (Bethesda, MD, USA), U01 GM074492, funded as part of the Pharmacogenomics Research Network. This work is also supported by the following Grants from the NIH National Center for Research Resources: Grant M01 RR00082 and UL1 RR029890 to the University of Florida, Grants UL1 RR025008 and M01 RR00039 to Emory University and UL1 RR024150 to Mayo Clinic. Support was also provided by K23 HL086558 (RMC), K23 HL091120 (ALB) and T32 DK007518 (JDD). The authors are thankful for the technical assistance of Zhiying Wang and Lynda Stauffer. The authors acknowledge and thank the valuable contributions of the study participants, support staff and study physicians.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the The Pharmacogenomics Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Duarte, J., Turner, S., Tran, B. et al. Association of chromosome 12 locus with antihypertensive response to hydrochlorothiazide may involve differential YEATS4 expression. Pharmacogenomics J 13, 257–263 (2013). https://doi.org/10.1038/tpj.2012.4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tpj.2012.4
Keywords
This article is cited by
-
Genome Wide Analysis Approach Suggests Chromosome 2 Locus to be Associated with Thiazide and Thiazide Like-Diuretics Blood Pressure Response
Scientific Reports (2019)
-
Hypertension pharmacogenomics: in search of personalized treatment approaches
Nature Reviews Nephrology (2016)
-
The effects of genes implicated in cardiovascular disease on blood pressure response to treatment among treatment-naive hypertensive African Americans in the GenHAT study
Journal of Human Hypertension (2016)
-
An update on the pharmacogenetics of treating hypertension
Journal of Human Hypertension (2015)
-
Pharmacogenomics of Hypertension and Heart Disease
Current Hypertension Reports (2015)