Abstract
Ficin is classified as a sulfhydryl protease isolated from the latex of fig trees. In most cases, a particular enzyme fits a few types of substrate and catalyzes one type of reaction. In this investigation, we found sufficient proofs for the intrinsic peroxidase-like activity of ficin and designed experiments to examine its effectiveness in a variety of scenarios. Ficin can transform peroxidase substrates to colored products in the existence of H2O2. Our results also indicate that the active sites of peroxidase-like activity of ficin are different from that of protease, which reveals that one enzyme may catalyze more than one kind of substrate to perform different types of reactions. On the basis of these findings, H2O2 releasing from MCF-7 cells was detected successfully. Our findings support a wider application of ficin in biochemistry and open up the possibility of utilizing ficin as enzymatic mimics in biotechnology and environmental monitoring.
Similar content being viewed by others
Introduction
Enzymes are indispensable biological catalysts for self-replication and for the metabolism of organisms. They are the most specific catalysts known1, both from the viewpoint of the substrate and the type of reaction performed on the substrate2. In most cases, a particular enzyme fits a few types of substrate and catalyzes one type of reaction. One exception is DNA polymerases, which are widely used in polymerase chain reaction (PCR), possess both polymerase activity and exonuclease activity. These different activities are often located in separately structured domains on the same polypeptide chain3,4. However, reports of enzymes catalyzing more than one type of reaction are very scarce.
Peroxidases (POXs, EC 1.11.1.x) are a large family of enzymes, found extensively in animals, plants, and microorganisms. Class III plant peroxidase (POX, EC 1.11.1.7), a plant-specific oxidoreductase and heme-containing glycoprotein5, plays a part in increasing the plant defenses against pathogens6. Peroxidase has a ferriprotoporphyrin IX prosthetic group located at the active site. The major hallmark of this kind of enzymes is the ability to catalyze H2O2-dependent oxidoreduction, and reduce the toxicity of peroxides and some aromatic compounds (electron donors)7. They can also catalyze the conversion of chromogenic substrates into colored products that are detectable by spectrophotometric methods. Therefore, they have been widely applied to biochemical analyses8,9, such as western-blots10, enzyme-linked immunoabsorbent assay11 and immunohistochemistry12.
Horseradish peroxidase (HRP, EC1.11.1.7) is one of the most important peroxidases used in biochemical analysis. However, the applications of HRP are still limited because of its rigorous storage requirements, poor thermal stability, high expense, sensitivity to the environment and its short storage life due to denaturation and digestion. As a consequence, there is a good deal of current research interest in artificial enzyme mimics. To date, more and more mimetic enzymes have put to use, such as metal-oxides nanoparticles13,14, heme complex15,16, graphene oxide17,18, ionic nanoparticles19, carbon nanodots20, quantum dots21, and metal-organic frameworks22. These mimetic enzymes overcome the drawbacks of HRP and promote the development of artificial enzyme mimics. To one’s disappointment, however, some of these non-biological catalysts often need laborious preparation procedures and modification steps to suppress aggregation, which would result in low reproducibility and low catalytic activity. Biological catalysts, in comparison to non-biological catalysts, possess particularly high catalytic efficiency, high reaction rates under very mild and favorable biological reaction conditions. For this reason, it is still highly desirable to find biological peroxidase-like materials.
Ficin (EC 3.4.22.3), isolated from the latex of fig trees, is classified as a sulfhydryl protease. It cleaves proteins at the carboxyl side of glycine, serine, threonine, methionine, lysine, arginine, tyrosine, alanine, asparagine and valine. Ficin contains eight cysteine, and is stabilized by three disulfide bridges23. It is generally recognized that cysteine and histidine play a key role in the residues for the protease activity of ficin24,25,26. The sequence of amino acids around active sites has high degree of homology with the corresponding one in the cysteine protease papain27. Here we show our discovery that ficin possesses intrinsic peroxidase-like activity. And our results indicate that the active sites of peroxidase-like activity of ficin are different from that of protease. Our findings reveal that one enzyme may catalyze more than one kind of substrates to perform different type of reactions. On the basis of these findings, H2O2 releasing from MCF-7 cells was detected successfully.
Results and Discussion
Discovery of intrinsic peroxidase-like activity of ficin
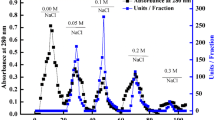
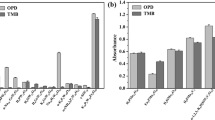
Nature peroxidases, such as HRP, show strong catalytic activity and substrate specificity in the transformation of chromogenic substrates to colored products in the existence of H2O228. Herein, we found sufficient proof for intrinsic peroxidase-like activity of ficin. As shown in Figure 1a, in different reaction systems, 3,3′,5,5′-tetramethylbenzidine (TMB), the typical substrate for peroxidases, was oxidized in the existence of H2O2 only when ficin was added, resulting in a blue reaction product and a maximum absorbance at 652 nm29. This result showed that the peroxidase-like activity toward TMB came from the robust intrinsic catalytic property of ficin. To further verify the peroxidase-like activity of ficin, we performed experiments using other peroxidase substrates instead of TMB, including o-phenylenediamine (OPD) and 2,2′-azino-bis (3-ethylbenzthiazo-line-6-sulfonic acid) diammonium salt (ABTS). We found that ficin catalyzed the oxidation of TMB, OPD and ABTS by H2O2 in pH 5.0 PBS buffer, followed the expected typical color changes (Figure S1). By comparison, ficin or H2O2 alone could not produce significant color change. These results confirm that ficin possesses the capability to catalyze oxidation of organic substrates and exhibits an intrinsic peroxidase-like activity.
(a) The absorption spectra of TMB in different reaction systems: TMB+Ficin (1), TMB + H2O2 (2), and TMB+H2O2 + Ficin (3) in 20 mM PBS buffer (pH 5.0) at 35 °C after 2 hour incubation. The concentrations were 0.10 μg mL−1 for ficin, 0.80 mM for H2O2 and TMB. The inset shows corresponding digital image. (b) EPR spectra of •OH radicals in the system of DMPO, DMPO-H2O2, DMPO-H2O2-Ficin (0.10 μg mL−1) and DMPO-H2O2-Ficin (0.50 μg mL−1).
The intrinsic peroxidase-like activity of ficin can be further certified by the EPR experiment. 5,5-Dimethyl-1-pyrroline-N-oxide (DMPO), a widely used hydroxyl radical trapping reagent, is applied to confirm the generation of DMPO/•OH spin adduct with high sensitivity and selectivity. As Fig. 1b shows, the typical DMPO/•OH spin adduct signal intensity in the systems of DMPO and DMPO-H2O2 are not significant when compared to DMPO-H2O2-ficin, demonstrating that ficin converts H2O2 to •OH radical29. In addition, the typical DMPO/•OH spin adduct signal intensity was increased by the increase in the concentration of ficin, demonstrating that the peroxidase-like activity of ficin may originate from the catalytic ability to convert H2O2 to •OH radical. The results of EPR provide empirical evidence that ficin possesses intrinsic peroxidase-like activity.
pH, temperature, substrate concentrations, and incubation time dependence
Similar to HRP, the peroxidase-like activity of ficin is also dependent on experimental conditions. We explored the effect of pH, temperature, H2O2 concentration, TMB concentration and reaction time on the relative activity (Fig. 2 and Figure S2). At a weakly acidic pH, ficin possesses higher catalytic activity, and the maximal relative activity of ficin was found at pH 5.0 (Fig. 2a). The effect of H2O2 concentration on relative activity was tested in the range of 0.10 to 5.0 mM, and the relative activity increased with an increasing H2O2 concentration in the range from 0.10 to 0.80 mM. With a concentration of H2O2 higher than 0.80 mM, the relative activity decreased (Fig. 2b). Higher H2O2 concentrations will inhibit the peroxidase-like activity of ficin, and this behavior is similar to HRP30. As shown in Fig. 2c and d, the optimal temperature and reaction time for the maximum relative activity of ficin were 35 °C and 2 hours. The effect of the concentration of TMB was also investigated. As shown in Figure S2, the optimal TMB concentration for maximum relative activity of ficin was 0.80 mM. Thus, the optimal pH, temperature, H2O2 concentration, TMB concentration and reaction time were as follows: 5.0, 35 °C, 0.80 mM, 0.80 mM, 2 hours. These optimum parameters were very similar to those observed with HRP, but different from those of protease31.
The peroxidase-like activity of ficin is pH (a), H2O2 concentration (b), temperature (c), and incubation time (d) dependent. Experiments were carried out using 0.10 μg mL−1 ficin or 0.10 ng mL−1 HRP with 0.80 mM TMB as substrate, respectively. The H2O2 concentration was 0.80 mM for ficin and 1.0 mM for HRP. The pH was 5.0, and the temperature was 35 °C unless otherwise stated. For each curve, the maximum point was defined as 100% and error bars represent the standard deviations of three independent experiments.
Michaelis constant determination and catalytic mechanism
Aiming at measuring the catalytic mechanism of ficin, the steady-state kinetic parameters for the oxidation of TMB in the presence of H2O2 were measured, and results were placed within a Michaelis-Menten kinetic model32. Within the suitable range of substrate (TMB and H2O2), typical Michaelis-Menten curves were obtained for both ficin (Fig. 3a,b) and HRP (Fig. 3c,d). The data were fitted well to the Michaelis-Menten model to acquire the enzyme kinetic parameters (Michaelis constant, maximum reaction rate, catalytic constant and catalytic efficiency) summarized in Table 1. We obtained the Km (TMB) of 0.19 mM, and the Km (H2O2) of 0.35 mM. Thus, the Km (TMB) for ficin was similar to that of HRP (0.15 mM), while the Km (H2O2) for ficin was 1.74 times lower than that of HRP (0.61 mM), suggesting that ficin has a higher affinity to H2O2. In addition, the catalytic efficiency (Kcat/Km) of ficin was calculated to be 5.89 mM−1 s−1 for TMB, and 2.31 mM−1 s−1 for H2O2. The catalytic efficiency of ficin were approximately 3 orders of magnitude lower than HRP. It may be due to the fact that ficin is a simple enzyme while HRP is a conjugated enzyme containing the ferroprotoporphyrin group.
(a–d) The velocity (v) of the reaction was measured using 1.0 μg mL−1 ficin (a,b) or 1.0 ng mL−1 HRP (c,d) under optimal conditions. Error bars represent the standard deviations of three independent experiments. (a,c) The concentration of H2O2 was 0.80 mM (ficin) or 1.0 mM (HRP) and varied concentration of TMB. (b,d) The concentration of TMB was 0.40 mM (ficin) or 0.60 mM (HRP) and varied concentration of H2O2. (e,f) Double-reciprocal plots of activity of ficin at a fixed concentration of one substrate versus varying concentration of the second substrate.
To further analyze the catalytic mechanism of ficin, double reciprocal plots of initial velocity catalytic mechanism were measured. Figure 3e and f show double reciprocal plots of initial velocity against one substrate concentration, obtained in a range of concentrations of the second substrate. Similar to HRP, the lines are mutually parallel, which is the characteristic of a Ping-Pong mechanism33. This finding clearly demonstrates that the catalytic mechanism of ficin is that ficin binds and reacts with the first substrate, then releases the first product before reacting with the second substrate.
Comparison of robustness of peroxidase-like activity of ficin and HRP
To investigate the pH and thermal robustness of peroxidase-like activity of ficin, ficin was first incubated in solution at a pH range (1–14) or a range of values of temperature (0–90 °C) for 2 hours. The peroxidase activity was tested under optimal conditions (pH 5.0 and 35 °C). For comparison, the catalytic activity of HRP was tested under same conditions, and the results were shown in Fig. 4a and b. Ficin showed robust peroxidase activity over a range of pH from 3.0 to 14.0, while the catalytic activity of HRP significantly decreased when the pH was lower than 4.0 or higher than 11.0, which is essentially in agreement with previous reports15,17. Meanwhile, ficin was found to possess outstanding thermal stability over a wide range of temperature from 0 to 70 °C, while the activity of HRP decreased by 70% over 50 °C. What is noteworthy is that this outstanding robustness of peroxidase-like activity of ficin against harsh pH and temperature suggest that it can be used more extensively in analytic applications than HRP.
Effects of pH (a) and temperature (b) on the robustness of ficin and HRP. 1.0 mg mL−1 ficin and 1.0 μg mL−1 HRP were first incubated at pH 1–14 for 2 h or 0–90 °C for 2 h. And then they were diluted to 0.10 μg mL−1 (ficin) or 0.10 ng mL−1 (HRP) to test the peroxidase activities under optimal conditions (20 mM PBS buffer, pH 5.0, 35 °C, 0.80 mM H2O2 and TMB). For each curve, the maximum point was defined as 100% and error bars represent the standard deviations of three independent experiments.
Peroxidase-like activity origins from ficin not impurities
It’s important to confirm that the observed peroxidase-like activity originated from ficin itself rather than the impurities coexisting in it. To rule out the possibility that the observed activity was caused by the peroxidase existing in ficin, highly purified premium grade ficin was used in our experiments. To further confirm it, 2× crystallized ficin were also used. After the removal of cysteine, which was added by the supplier to activate the protease activity of ficin, 2× crystallized ficin can also catalyze the oxidation of TMB by H2O2 (Figure S3). These results show that the peroxidase-like activity is not due to coexisting peroxidase. The robustness of peroxidase-like activity of ficin against harsh pH and temperature was also a collateral to support this conclusion.
Some metal ions can catalyze the chromogenic reaction between TMB and H2O2. To test the metal content of ficin, ICP-MS (Agilent 7700ce) was used. As illustrated in Table S1, the metal concentrations were less than 10 nM: Al (9.63 nM), Mg (9.17 nM), Zn (7.69 pM), Ca (2.15 nM), Fe (0.04 nM), K (2.08 nM). Additionally, we tested the catalytic effect of segmental metal ions coexisting in ficin under standard conditions (0.20 M PBS buffer, pH 5.0, 35 °C). It was found that the catalytic activity of metal ions (100 nM, 10 nM, 1 nM) was insignificant compared with ficin (Figure S4a), which supports our suggestion that the peroxidase-like activity originates from ficin rather than from the metal ions coexisting in ficin. Additionally, ethylenediaminete-traacetic acid (EDTA) was added into the reaction solution. Even though EDTA was added at concentrations up to 1.0 mM, the peroxidase-like activity of ficin did not change (Figure S4b), which further rules out the effect of metal ions. These results verified the peroxidase-like activity is due to ficin not impurities.
Effects of mercuric chloride and iodoacetic acid on the peroxidase-like activity of ficin
A reduced -SH group is necessary for the protease activity of ficin34, and this activity can be inhibited by mercuric chloride and iodoacetic acid due to the binding to cysteine35, one of the active sites of ficin when it acts as a protease27. Herein, we tested effects of mercuric chloride and iodoacetic acid on the peroxidase-like activity of ficin. However, neither 100 μM of mercuric chloride nor 100 μM of iodoacetic acid inhibited the peroxidase-like activity of ficin (Figure S5). This result indicates that the active sites of peroxidase-like activity are different from that of protease.
As a protease, ficin contains about 200 amino acids, and its catalytic dyad is made up of cysteine and histidine. Why did ficin develop a polypeptide chain with more amino acids than necessary during the long-term natural evolution? Except in the disulfide bridges and active sites, what is the function of other amino acids? Peroxidases are known to play a part in increasing a plant’s defenses against pathogens and reducing the toxicity of some organic substrates6. Hence, it is logical to discover that a protease possesses peroxidase-like activity.
Ficin applied to detect the H2O2 releasing from living cells
The color variation of TMB oxidation catalyzed by ficin was H2O2 concentration-dependent. This indicates that the absorbance change can be used for the detection of H2O2 (Fig. 5a). Based on the peroxidase-like activity of ficin, H2O2 releasing from MCF-7 cells was detected successfully (Fig. 5b). When the MCF-7 cells were stimulated by 10 mM n-butyric acid, 1.96 μΜ H2O2 releasing occurred, calculated to be 9.80 × 10−15 mol/cell. This value matches with that reported previously36,37. To evaluate effects of other stimuli, the amount of H2O2 releasing from MCF-7 cells induced by adenosine-5-diphosphate (ADP, 200 ng mL−1), ascorbic acid (AA, 200 ng mL−1), n-formylmethionyl-leucylphenyl-alanine (fMLP, 200 ng mL−1) were also measured. As shown in Fig. 5B, the highest amount of H2O2 releasing occurred by using 10 mM n-butyric acid as stimuli.
(a) Linearity of absorbance against a H2O2 concentration range of 0–20 μM. (b) Comparison of fluxes of H2O2 release from cells induced by ADP (200 ng mL−1, 0.47 μM), AA (200 ng mL−1, 1.14 μM), fMLP (200 ng mL−1, 0.46 μM) and n-butyric acid (10 mM). All the conditions: 0.10 μg mL−1 ficin, 0.80 mM TMB, 20 mM PBS buffer (pH 5.0), and 35 °C. Error bars represent the standard deviations of three independent experiments.
Conclusions
In summary, we found sufficient proofs for the intrinsic peroxidase activity of ficin, which catalyzed the reaction of different peroxidase substrates in the presence of H2O2. In addition, the active sites of peroxidase-like activity of ficin are different from those of protease, which reveals that one enzyme may catalyze more than one kind of substrate to perform different types of reactions. The peroxidase-like activity of ficin was dependent on pH, temperature, H2O2 concentration, incubation time, and showed typical Michaelis-Menten kinetics with a Ping-Pong mechanism. Based on the peroxidase-like activity of ficin, H2O2 releasing from MCF-7 cells was detected successfully. We argue that the peroxidase-like activity of ficin may play a part in defending pathogens and reducing toxicity of some organic compounds. Our findings support a wider application of ficin in biochemistry and open up the possibility of utilizing ficin as enzymatic mimics in biotechnology and environmental monitoring.
Methods
Reagents and materials
3,3′,5,5′-tetramethylbenzidine (TMB), o-phenylenediamine (OPD) and 2,2′-azino-bis (3-ethylbenzo-thiazoline-6-sulfonic acid) diammonium salt (ABTS) were obtained from Sangon Biotech Co. Ltd. Premium grade ficin (F4165, powder, molecular weight 23.8 kDa), 2× crystallized ficin (F4125, saline suspension, containing 30 mM cysteine) and horseradish peroxidase (HRP, 300 U mg−1) were purchased from Sigma-Aldrich. H2O2 was obtained from Chongqing Pharmaceutical Co., Ltd. N-butyric acid, adenosine- 5-diphosphate (ADP), ascorbic acid (AA), and n-formylmethionyl-leucyl-phenylalanine (fMLP) were obtained from Aladdin. Ultrapure water (18.2 MΩ) was prepared with a Milli-Q system and used in all experiments. A NaH2PO4-Na2HPO4 buffer solution (PBS, 20 mM, pH from 1.0–12.0) was used in this study, and the pH of buffer solutions was adjusted with H3PO4 or NaOH (20 mM) solution. Additionally, for pH 13 or 14, KOH solutions were used to replace corresponding buffer solutions.
Electron paramagnetic resonance (EPR)
Samples were prepared at room temperature by adding 4.0 mM H2O2, and 25.0 mM DMPO with different concentrations of ficin, and then adding a 20 mM PBS buffer (pH = 5.0) into a plastic tube. Subsequently the prepared sample solution was transferred to a quartz capillary tube and placed in the EPR cavity. Spectra were recorded afterwards. DMPO was used to trap the •OH radicals to form the DMPO/•OH spin adduct. The EPR spectra were obtained on a JESFA200 (JEOL, Japan) with a microwave bridge (modulation width, 0.2 mT; modulation amplitude, 2 Gauss; frequency power, 1 mW; modulation frequency, 100 kHz).
Kinetic analysis
Steady-kinetic measurements were carried out by monitoring the change in absorbance at 652 nm on a microplate reader (Infinite 200 PRO, TECAN, Austria). Experiments were carried out using 1.0 μg mL−1 ficin or 1.0 ng mL−1 HRP in 20 mM PBS buffer (pH 5.0) at 35 °C with 0.80 mM H2O2 for ficin, 1.0 mM for HRP or 0.40 mM TMB for ficin, 0.60 mM for HRP as substrate, unless otherwise stated. Double-reciprocal plots of activity of ficin were obtained at a fixed concentration of one substrate versus varying concentrations of the second substrate. The Michaelis–Menten constant was calculated using Lineweaver–Burk plots of the double reciprocal of the Michaelis−Menten equation, 1/ν = Km/Vmax⋅(1/[S]+1/Km), where v is the initial velocity, Vmax is the maximal reaction velocity, [S] is the concentration of substrate, Km is the Michaelis constant.
All other spectroscopy measurements were carried out by recording the absorbance change at 652 nm with a UV-Vis spectrophotometer (UV-2450, Shimadzu, Japan).
Removal of cysteine existing in 2×crystallized ficin
To active the protease activity of ficin, 30 mM of cysteine was added in 2× crystallized ficin by the supplier. However, cysteine can inhibit the peroxidase-like activity of ficin drastically. Therefore, we removed cysteine by centrifugal ultrafiltration or dialysis before investigating the peroxidase-like activity of 2× crystallized ficin. They were both suspended in a 10-fold dilution saline suspension. Then the exact concentration of ficin was estimated based on percentile absorptivity E1% = 21.0 (280 nm). Centrifugal ultrafiltration (ultra-filtration tubes with cutoff molecular weight of 5 kDa) was carried out at 4 °C, 3,000 rpm for 10 minutes each time and repeated 10 times just before use. The stock solution of ficin was dialyzed (dialysis bags with cutoff molecular weight of 5 kDa) in ultra-pure water for 48 hours at 4 °C and the ultra-pure water was changed every two hours.
Culture of MCF-7 cell
MCF-7 cell (human breast cancer cell) lines purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai) were used as target cells. Cells were incubated in DMEM containing 10% fetal bovine serum (FBS, Gicbo), 100 U mL−1 of penicillin and 100 μg mL−1 streptomycin. They were maintained at 37 °C in a humidified and 5% CO2 incubator. The cell density was determined using a Scepter handheld automated cell counter (Millipore), and this was performed prior to experiments.
Measurement procedure of H2O2
For measuring the H2O2 standard solution, the H2O2 concentration-dependent absorbance was studied. In a typical process, 0.10 μg mL−1 ficin and 0.80 mM TMB were added in 96-well plate, after which several different concentrations of H2O2 was added, and then the above mixture was incubated in 20 mM PBS buffer (pH 5.0) at 35 °C for 2 hours to allow development of the blue color. Next, the absorbance at 652 nm of each well was determined immediately by a microplate reader (Infinite 200 PRO, TECAN, Austria).
To measure the H2O2 releasing from living cells, MCF-7 cancer cell was cultured in 96-well plate with cell density of 2.0 × 105 cells mL−1 for 24 hours. After the cultural medium was removed, 10 mM n-butyric acid was added, and this mixture was incubated for 1 min by shaking. Then 0.10 μg mL−1 ficin, 0.80 mM TMB, 20 mM PBS buffer (pH 5.0) were further added (a final volume of 200 μL) and incubated at 35 °C for 2 hours to finish the reaction. The absorbance was determined by a microplate reader. H2O2 releasing from MCF-7 cells induced by ADP (200 ng mL−1, 0.47 μM), AA (200 ng mL−1, 1.14 μM), fMLP (200 ng mL−1, 0.46 μM) were also measured, and compared with those induced by n-butyric acid (the number of cells used in the measurements is ~4 × 105 cells).
Additional Information
How to cite this article: Yang, Y. et al. Intrinsic Peroxidase-like Activity of Ficin. Sci. Rep. 7, 43141; doi: 10.1038/srep43141 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Schellenberger, V., Siegel, R. A. & Rutter, W. J. Analysis of enzyme specificity by multiple substrate kinetics. Biochemistry 32, 4344–4348 (1993).
Baggott, J. Textbook of Biochemistry: with Clinical Correlations (3rd ed., ed. Devlin, T. M. ) 167–168 (Wiley-Liss: New York, 1992).
Murphy, D. L., Jaeger, J. & Sweasy, J. B. A triad interaction in the fingers subdomain of DNA polymerase beta controls polymerase activity. J. Am. Chem. Soc. 133, 6279–6287 (2011).
Freeman, R., Liu, X. & Willner, I. Amplified multiplexed analysis of DNA by the exonuclease III-catalyzed regeneration of the target DNA in the presence of functionalized semiconductor quantum dots. Nano lett. 11, 4456–4461 (2011).
Hiraga, S., Sasaki, K., Ito, H., Ohashi, Y. & Matsui, H. A large family of class III plant peroxidases. Plant cell physiol. 42, 462–468 (2001).
Karthikeyan, M. et al. Induction of resistance in host against the infection of leaf blight pathogen (Alternaria palandui) in onion (Allium cepa var aggregatum). Indian. J. Biochem. Bio. 42, 371–377 (2005).
Hinman, R. L. & Lang, J. Peroxidase-catalyzed oxidation of indole-3-acetic acid*. Biochemistry 4, 144–158 (1965).
Ryan, B. J., Carolan, N. & Ó’Fágáin, C. Horseradish and soybean peroxidases: comparable tools for alternative niches? Trends biotechnol. 24, 355–363 (2006).
Klibanov, A. M., Tu, T. M. & Scott, K. P. Peroxidase-catalyzed removal of phenols from coal-conversion waste waters. Science 221, 259–261 (1983).
Towbin, H., Staehelin, T. & Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76, 4350–4354 (1979).
Yu, X. et al. Carbon nanotube amplification strategies for highly sensitive immunodetection of cancer biomarkers. J. Am. Chem. Soc. 128, 11199–11205 (2006).
Budwit-Novotny, D. A. et al. Immunohistochemical analyses of estrogen receptor in endometrial adenocarcinoma using a monoclonal antibody. Cancer Res. 46, 5419–5425 (1986).
Gao, L. et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nature Nanotechnol. 2, 577–583 (2007).
Zeb, A. et al. Highly Efficient Fenton and Enzyme-Mimetic Activities of Mixed-Phase VOx Nanoflakes. ACS Appl. Mater. Interfaces 8, 30126−30132.
Atamna, H. & Boyle, K. Amyloid-β peptide binds with heme to form a peroxidase: Relationship to the cytopathologies of Alzheimer’s disease. Proc. Natl. Acad. USA 103, 3381–3386 (2006).
Huang, Y., Lin, Y., Ran, X., Ren, J. & Qu, X. Self-Assembly and Compartmentalization of Nanozymes in Mesoporous Silica‐Based Nanoreactors. Chem. Eur. J. 22, 5705–5711 (2016).
Song, Y., Qu, K., Zhao, C., Ren, J. & Qu, X. Graphene oxide: intrinsic peroxidase catalytic activity and its application to glucose detection. Adv. Mater. 22, 2206–2210 (2010).
Yang Z. et al. A facile label-free colorimetric aptasensor for acetamiprid based on the peroxidase-like activity of hemin-functionalized reduced graphene oxide. Biosens. Bioelectron. 65, 39–46 (2015).
Liu, Y. et al. Ionic Functionalization of Hydrophobic Colloidal Nanoparticles To Form Ionic Nanoparticles with Enzymelike Properties. J. Am. Chem. Soc. 137, 14952–14958 (2015).
Shi, W. et al. Carbon nanodots as peroxidase mimetics and their applications to glucose detection. Chem. Commun. 47, 6695–6697 (2011).
Peng H. et al. Fabrication and multifunctional properties of ultrasmall water-soluble tungsten oxide quantum dots. Chem. Commun. 52, 9534–9537 (2016).
Zhao, M. et al. Core–shell palladium nanoparticle@ metal–organic frameworks as multifunctional catalysts for cascade reactions. J. Am. Chem. Soc. 136, 1738–1741 (2014).
Englund, P. T., King, T. P., Craig, L. C. & Walti, A. N. D. A. Ficin. I. Its isolation and characterization. Biochemistry 7, 163–175 (1968).
Husain, S. S. & Lowe, G. The amino acid sequence around the active-site cysteine and histidine residues, and the buried cysteine residues in ficin. Biochem. J. 117, 333–340 (1970).
Friedenson, B. & Liener, I. E. The active site sequence of multiple forms of ficin. Arch. Biochem. Biophys. 149, 169–174 (1972).
Liener, I. E. & Friedenson, B. [17] Ficin. Meth. Enzymol. 19, 261–273 (1970).
Lowe, G. The cysteine proteinases. Tetrahedron 32, 291–302 (1976).
Josephy, P. D., Eling, T. & Mason, R. P. The horseradish peroxidase-catalyzed oxidation of 3,5,3′,5′-tetramethylbenzidine. Free radical and charge-transfer complex intermediates. J. Biol. Chem. 257, 3669–3675 (1982).
Marquez, L. A. & Dunford, H. B. Mechanism of the oxidation of 3,5,3′,5′-tetramethylbenzidine by myeloperoxidase determined by transient-and steady-state kinetics. Biochemistry 36, 9349–9355 (1997).
Nicell, J. A. & Wright, H. A model of peroxidase activity with inhibition by hydrogen peroxide. Enzyme Microb. Tech. 21, 302–310 (1997).
Devaraj, K. B., Kumar, P. R. & Prakash, V. Purification, characterization, and solvent-induced thermal stabilization of ficin from Ficus carica. J. Agr. Food Chem. 56, 11417–11423 (2008).
Purich, D. L. Contemporary Enzyme Kinetics and Mechanism (3rd edition) 280–289 (Beijing: Science Press, 2009).
Porter, D. & Bright, H. The mechanism of oxidation of nitroalkanes by horseradish peroxidase. J. Biol. Chem. 258, 9913–9924 (1983).
Bernhard, S. A. & Gutfreund, H. Ficin-catalysed reactions: the affinity of ficin for some arginine derivatives. Biochem. J. 63, 61 (1956).
Kortt, A. A., Hamilton, S., Webb, E. C. & Zerner, B. Ficins (EC 3.4. 22.3). Purification and characterization of the enzymic components of the latex of Ficus glabrata. Biochemistry 13, 2023–2028 (1974).
Wu, P., Qian, Y., Du, P., Zhang, H. & Cai, C. Facile synthesis of nitrogen-doped graphene for measuring the releasing process of hydrogen peroxide from living cells. J. Mater. Chem. 22, 6402–6412 (2012).
Shi Q. et al. Mesoporous Pt Nanotubes as a Novel Sensing Platform for Sensitive Detection of Intracellular Hydrogen Peroxide. ACS Appl. Mater. Interfaces 7, 24288–24295 (2015).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21175110, No. 21405124), the Fundamental Research Funds for the Central Universities (No. XDJK2013A022).
Author information
Authors and Affiliations
Contributions
H.Z. and Z.X. conceived and designed the research. Y.Y., D.S. performed experiments, Y.L. contributed new reagents and analyzed the data. Y.Y. and H.Z. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Yang, Y., Shen, D., Long, Y. et al. Intrinsic Peroxidase-like Activity of Ficin. Sci Rep 7, 43141 (2017). https://doi.org/10.1038/srep43141
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep43141
This article is cited by
-
Bromelain and ficin proteolytic effects on gliadin cytotoxicity and expression of genes involved in cell-tight junctions in Caco-2 cells
Amino Acids (2023)
-
Ficin-copper hybrid nanoflowers with enhanced peroxidase-like activity for colorimetric detection of biothiols
Microchimica Acta (2023)
-
Inhibitory effect of ficin on Candida albicans biofilm formation and pre-formed biofilms
BMC Oral Health (2022)
-
Comparative stability of ficin and papain in acidic conditions and the presence of ethanol
Amino Acids (2019)
-
Inhibitory Effect of Ficin Derived from Fig Latex on Inflammation and Melanin Production in Skin Cells
Biotechnology and Bioprocess Engineering (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.