Abstract
We studied germination behaviors and persistence mechanism of wild Glehnia littoralis, a typical coastal species at temperate sandy coasts of the North Pacific Ocean, and tested the hypothesis that the coastal plants may have evolved special seeds adapting to the coasts, by which they recruit and persist easily, occupying the coasts as ideal habitats. In the Shandong Peninsula, China, we investigated temperature and moisture conditions of coast sand in relation to germination and evaluated effects of sand burial, seawater immersion and sowing time on germination. When germination began, daily dawn temperatures of sand were about 10 °C and daily noon temperatures were about 25 °C; the temperatures were not different in the sand <8 cm deep. The sand at these depths showed a significant difference in moisture contents. The seeds exhibited large germination rates if sand burial was at depths >= 3 cm and winter freezing was kept longer than 2.5 months. Seeds experiencing seawater immersion were able to germinate well. These evidences suggest that G. littoralis has evolved special seeds adapting to seawater dispersal and specific season rhythm. By the seeds, G. littoralis occupies temperate sandy coasts as ideal habitats to persist.
Similar content being viewed by others
Introduction
The worldwide coastal vegetation suffers from increasing habitat loss, tourist trampling and artificial dams1,2,3. Many coastal species making up the vegetation are declining, and local or global extinction is ongoing4,5. They are irreplaceable stabilizers or maintainers of coastal ecosystems, and also rare resources to provide humans with herbal medicine, fibre and other necessities6,7,8. Glehnia littoralis that can be used as herbal medicine to treat lung diseases of humans is a typical species growing at temperate sandy coasts around the North Pacific Ocean9,10,11,12. Unfortunately, it is now in danger of extinction for habitat loss and exploitation13,14,15. Human efforts are needed to save the species from extinction. Such coastal specialists may have established special relationships with pertaining coasts16,17. Knowledge of these relationships is useful for making a plan of species conservation18,19.
Wild G. littoralis is distributed from Eastern China, the Korea peninsula to Western Canada and America7,16,20. It is not a dominant species of coastal vegetation, and the distribution is narrowly limited to supratidal zones of fore-dunes in widths of ca. 10 or 20 m16,21. Adults of the species are often not taller than 40 cm, with annual leaves and fruiting branches, and with perennial roots and short stems. The roots can grow deeper than 40 or 50 cm to survive fierce winter. Several decades ago, the species was widespread at fore-dunes of sandy coasts, but at present, is very rare and endangered, only appearing at a few remote coasts free of tourism and exploitation15,21. The Chinese government has enrolled it as a species deserving strict conservation, and because of significant effects in curing lung diseases, it has been transplanted inland for field cultivation12,22,23,24,25. Nevertheless, we doubt that the ex situ cultivation can provide the species with ideal conditions for persistence.
Supratidal zones of temperate sandy coasts are characterized by environmental factors such as rich sand matrix, long winter and, in particular, occasional inundation during seawater surges26,27,28. As a survivor with long history, G. littoralis may have adapted to these factors and evolved a special life7,17. The life of a plant species always involves seeds and seedlings29,30,31. G. littoralis seeds were found to be buoyant13,17,32,33. However, persistence of wild G. littoralis was unknown. Given this background, we designed field surveys and experiments to recognize germination behaviors and persistence mechanism of wild G. littoralis. Furthermore, we tested the hypothesis that the typical coastal species has evolved special seeds to adapt to temperate coasts, by which it recruits and persists easily, occupying temperate sandy coasts as ideal habitats.
Results
Temperature and moisture conditions for germination
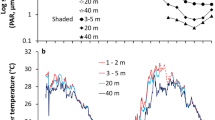
When the germination began, daily dawn and noon temperatures of the sand were ca. 10 °C and 25 °C, respectively. The germination lasted for about a month (Fig. 1). During the process, the daily dawn and noon temperatures were 12.9 (mean) ± 2.1 (standard deviation) °C and 26.9 ± 8.6 °C, respectively. The maximum daily dawn temperature was 16 °C, and the maximum daily noon temperature was 40 °C. Two-way ANOVA indicated that these temperatures were not different among the four layers, but varied significantly with time changing (Table 1).
The results of two-way ANOVA indicated that moisture contents varied significantly with time, and nearly significantly among the four layers (Table 1). The moisture contents were 0.055 (mean) ± 0.076 (standard deviation) at the 0–2 cm layer, with a variation coefficient of 1.374 (standard deviation/mean); 0.071 ± 0.071 at the 2–4 cm layer, with a variation coefficient of 1.000; 0.084 ± 0.072 at the 4–6 cm layer, with a variation coefficient of 0.848; and 0.076 ± 0.046 at the 6–8 cm layer, with a variation coefficient of 0.608. The top layer was the driest and most unsteady, perhaps because of intense and variable evaporation. The 6–8 cm layer was not the moistest perhaps because rains during the early spring were often too weak to infiltrate so deep.
Effects of burial depths, seawater immersion and sowing time on germination
The cumulative germination rates responded differently to the burial depths (Table 1). The germination rates were significantly low at the depths <= 2 cm (Fig. 1), but were fairly high at the depths >= 3 cm. These results demonstrate that the depths favorable for germination were not less than 3 cm, perhaps because moisture contents were high and steady at these depths.
Seawater immersion might have no effects on final cumulative germination rates (Table 1), which could exceed 60%, but dynamics of the germination differed obviously among these immersion levels (Fig. 2). These results indicate that seawater immersion might not promote the maximum germination rates of G. littoralis seeds but could activate the seeds for a swift germination. The pre-germination immersion for 25 days was the best in urging the germination.
Sowing time dominated the cumulative germination rates (Table 1). The earlier the seeds were sown in winter, the higher the final cumulative germination rates (Fig. 3). Outdoor freezing for longer than 2.5 months was necessary to arouse G. littoralis seeds for a swift and successful germination. The finding suggests that G. littoralis seeds have the property of winter-dormancy, and most of them remain dormant until winter provides them a freezing longer than 2.5 months.
Discussion
Seeds of wild G. littoralis require appropriate temperatures and moisture for germination. After daily dawn and noon temperatures of sand rise to ca. 10 and 25 °C, G. littoralis seeds get ready for germination. We observed that if sown at the depths >= 3 cm, they germinate easily. The four sand layers showed a significant difference in moisture contents, other than in temperatures. Moisture contents were the lowest and most unstable at the 0–2 cm depth, which was adverse to germination. At the depth >= 3 cm, moisture contents became high and steady adequately, feasible for seed germination.
It was reported on that seeds of G. littoralis have the property of winter-dormancy13,34. This study provides details to further understand this. In wilderness, seeds of wild G. littoralis can hardly germinate unless aroused by fierce freezing longer than 2.5 months. This property is a protective adaptation of this species to the long winter of the temperate zone. Adults of the species bear seeds in late summer, when temperatures are favorable. Nevertheless, the seeds refuse to germinate that time. Because of winter-dormancy, they choose to germinate after winter, thus new seedlings emerge in early spring, gaining more time to grow strong to survive the coming winter.
Glehnia littoralis seeds can keep vigorous or turn more vigorous after seawater immersion, unlike most terrestrial plants35,36,37. This is a critical adaptation to seawater dispersal33,38,39. Supratidal zones are characterised by occasional seawater inundation8,40. Around the North Pacific Ocean, extreme storms often take place around winter to cause violent seawater surges41,42. As a result, seeds of G. littoralis at supratidal zones can be inundated and dispersed17,43,44. They keep afloat for many days, like seeds of Eryngium maritimum, Cakile maritime and Canavalia rosea17,38,39. They are immersed in seawater, whereas await a chance to land at supratidal zones again17. After they land at favorable environment, they may germinate and grow, continuing life cycles of the species. In case G. littoralis seeds were disabled during the immersion, this species would have difficulties in recruiting, and not persist for long along supratidal zones.
These germination behaviors confirm the hypothesis that the typical coastal specialist G. littoralis have evolved special seeds to adapt to temperate sandy coasts, at least including seawater dispersal, season rhythm and sandy matrix. By coupling the germination with the environment, we conceived the mechanism of wild G. littoralis for dispersal and persistence along temperate sandy coasts.
Adults of wild G. littoralis give birth to mature seeds before autumn. Although temperatures remain favorable that time, inherent dormancy sets back immediate germination of them. Storms can take place around winter in temperate seas, unlike in tropical ones42,45. They inevitably cause seawater surging, which may co-occur and overlap with high astronomical tides to make extreme surges. The surges can inundate supratidal zones for several hours. On this occasion, G. littoralis seeds are immersed in seawater and begin dispersing with seawater current17,46. Because of high buoyancy, they keep afloat during the immersion, and are able to return to supratidal zones with the same surge or another surge some days later17,47. With seawater, they disperse in short and long distances, and may settle at supratidal zones again. After settling at supratidal zones of temperate sandy coasts, they get the opportunity to began new lives. They are buried by moving sand with winds. During the burial, fierce winter provides them with effective freezing. After winter, they get aroused and ready for germination. In early spring, the daily dawn temperature rises to ca. 10 °C, and some seeds receive the signal to germinate48. Those on or in surface sand (< 3 cm deep) can hardly germinate, perhaps because of unfavorable moisture conditions. From spring to autumn, winds are relatively mild and temperatures are favorable, thus the seedlings grow as fast as possible. Several months later, taproots of them grow deep and strong enough to tolerate the first winter they will encounter. After the winter, they revive to grow. By perennial roots and winter-resistant buds, they repeat the revival and growth year after year, bearing seeds for species recruitment and persistence49.
The temperate sandy coasts around the North Pacific Ocean provide wild G. littoralis with ideal conditions, such as sand burial and freezing in windy winter, surging seawater for seed dispersal and activation, and calm growing season from spring to autumn. For such a provision, wild G. littoralis occupies temperate sandy coasts around the north Pacific Ocean as original habitats. After transplanted to inland, the species cannot disperse normally as along coasts, and has to lead a spoiled life, which may be adverse to species persistence50,51. Since G. littoralis is a typical coastal specialist depending on seawater dispersal, it ought to be conserved in temperate sandy coasts, where seawater dispersal is convenient, season rhythm is suitable and sand matrix is available. Additionally, with the warming of global climates, this species may shift its distribution area northward because long cold winter is necessary for it to germinate and regenerate, and it may be driven extinct from some warm temperate regions such as the Shandong Peninsula52.
Methods
Field surveys
Field surveys were conducted in 2012 at sandy coasts of the Shandong Peninsula (N 35.85–36.70°, E 120.05–121.20°, near Qingdao), where G. littoralis still exists along supratidal zone (Table 2). Seeds of the species germinated in early April. During the germination, we measured field temperatures of the sand with glass thermometers one time every 10 days, and the measurement lasted for a month until the germination almost ceased, i.e., four times in total. Daily dawn temperatures were measured just before sunrise, and daily noon temperatures were measured at 14:00. Each time of the measurement was done at three locations randomly chosen from an area of ca. 200 m2 where seedlings emerged in clumps, and at each location, four sand layers were investigated, covering the depths of 0–2, 2–4, 4–6 and 6–8 cm.
We sampled sand four times from these layers to measure moisture contents, one time every 10 days from the germination start. Every time, we randomly chose 10 points in the ca. 200 m2 area to sample sand. At these points, we collected sand vertically with a drill and divided the sand into the four layers. Sand from the same layer from the 10 points was together lumped and sealed in a waterproof plastic bag as a composed sample. Within 3 h, we weighed the samples and dried them in an electronic oven (70 °C for 2 days). We weighed the dried samples and calculated their gravimetric moisture contents, dividing the moisture mass (mass of the wet sample minus that of the dried sample) by the mass of the dried sample.
Experiments
In August 2012, we collected mature seeds of the species (carrying seed capsule) from undisturbed sandy coasts and air-dried them for storage. In November, we began the experiment one at a coast (E 120.93°, N 36.41°) to identify sand depths favorable for germination (Table 2). We loaded 20 wood boxes measuring 40 cm (length) × 20 cm (width) × 20 cm (height) with drainage holes at the bases with sand from the coast. These boxes were divided into five equal groups, one group containing four boxes. We sowed the seeds in these boxes, and sowing depths were one of the levels: 1, 2, 3, 4 and 5 cm. One box group (4 boxes) corresponded to one level of the sowing depths. In each box, 30 seeds were evenly sown at the equal depth. After sowing, we gently watered the sand within the boxes with collected rainfall. Then, we left the boxes outdoor and spread a mesh 2 m above to prevent birds (other than snow, rain and light). Monitoring was performed everyday. Since seedlings emerged, we counted new ones every 2 days, which we timely marked with fine toothpicks. The counting and recording lasted for a month until germination almost ceased.
In August 2013, we collected seeds again and air-dried them, to prepare the experiments two and three (Table 2). From October 17, we began to immerse seeds in seawater, one batch every 5 days. By November 16, we prepared seven batches of seeds ever immersed for 30, 25, 20, 15, 10, 5 and 0 days. During the immersing, we replaced the seawater every 5 days. On November 16, we fished out and sowed the seeds in the same boxes as the mentioned above, and placed them outdoor at the coast. Each immersion level included four boxes (replicates), where 30 seeds were evenly sown at the depth of 3 cm (we found it feasible for germination).
On November 20, 2013, the experiment three started to examine the effects of sowing time on germination (Table 2). We sowed the newly collected seeds but not subjected to any seawater immersion within the same boxes every month until March 20, 2014. The experiment included five levels of sowing time: November 20, December 20, January 20, February 20 and March 20. Every level included four boxes (as replicates), and in each box, 30 seeds were evenly sown at the depth of 3 cm. After sowing, we watered and monitored the boxes as in the experiment one. On April 6, 2014, germination began. Then, new seedlings were counted and marked every 2 days for a month. The authors comply with Convention on the Trade in Endangered Species of Wild Fauna and Flora.
Data analyses
Using two-way ANOVA, we examined the differences in daily dawn temperatures, daily noon temperatures and moisture contents among the four sand layers and the changing time points. The sand layers were four depth levels: 0–2, 2–4, 4–6 and 6–8 cm. The changing time points included four levels in the germination process: start of the germination, 1/3 of the germination period (the 10th day), 2/3 of the germination period (the 20th day) and the end of germination. We calculated cumulative germination rates over the germination process, which were seedling number until a certain day divided by the number of initially sown seeds. Using one-way ANOVA, we checked effects of sowing depth, seawater immersion and sowing time on the final cumulative germination rates. Data analyses were completed using Origin 8.0 (OriginLab, Northampton, USA) and R 3.2 (R Core Team 2015).
Additional Information
How to cite this article: Yang, H.-X. et al. Natural persistence of the coastal plant Glehnia littoralis along temperate sandy coasts. Sci. Rep. 7, 42784; doi: 10.1038/srep42784 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Acosta, A., Ercole, S., Stanisci, A. & Blasi, C. Sandy coastal ecosystems and effects of disturbance in Central Italy. J Coastal Res. 39, 985–989 (2006).
Lotze, H. K. et al. Depletion, degradation, and recovery potential of estuaries and coastal seas. Science. 312, 1806–1809 (2006).
Dugan, J. E. & Hubbard, D. M. Loss of coastal strand habitat in southern California: the role of beach grooming. Estuar Coast. 33, 67–77 (2010).
Duncan, R. P. & Young, J. R. Determinants of plant extinction and rarity 145 years after European settlement of Auckland, New Zealand. Ecology. 81, 3048–3061 (2000).
Defeo, O. et al. Threats to sandy beach ecosystems: a review. Estuarine, Coastal and Shelf Science. 81, 1–12 (2009).
Kathiresan, K., Boopathy, N. S. & Kavitha, S. Coastal vegetation–an underexplored source of anticancer drugs. Natural Product Radiance. 5, 115–119 (2006).
Voronkova, N. M., Burkovskaya, E. V., Bezdeleva, T. A. & Burundukova, O. L. Morphological and biological features of plants related to their adaptation to coastal habitats. Russ J Ecol+. 39, 1–7 (2008).
Yang, H. & Chu, J. Persistence of coastal vegetation in supratidal zones of northern China. Plos One. 8, e79964 (2013).
Masuda, T., Takasugi, M. & Anetai, M. Psoralen and other linear furanocoumarins as phytoalexins in Glehnia littoralis. Phytochemistry. 47, 13–16 (1998).
Hiraoka, N., Chang, J., Bohm, L. R. & Bohm, B. A. Furanocoumarin and polyacetylenic compound composition of wild Glehnia littoralis in North America. Biochem Syst Ecol. 30, 321–325 (2002).
Yuan, Z., Tezuka, Y., Fan, W., Kadota, S. & Li, X. Constituents of the Underground Parts of Glehnia littoralis. Chemical and Pharmaceutical Bulletin. 50, 73–77 (2002).
Cassileth, B. R. et al. Safety and pharmacokinetic trial of docetaxel plus an Astragalus-based herbal formula for non-small cell lung cancer patients. Cancer Chemoth Pharm. 65, 67–71 (2009).
Liu, Q., Hui, H. & Liu, M. Determination of seed vitality and germination rate of the endangered plant Glehnia littoralis. Journal of Plant Resources and Environment. 13, 55–56 (2003).
Murch, S. J., Peiris, S. E., Liu, C. & Saxena, P. K. In vitro conservation and propagation of medicinal plants. Biodiversity. 5, 19–24 (2004).
Song, C., Wu, B., Hu, J., Dong, Z. & Liu, Q. Existence status of Glehnia littoralis and causes of extinction in Jiangsu Province. Chinese Wild Plant Resources. 32, 56–57 (2013).
Kim, S., Shin, D., Song, H., Kim, S. & Yoon, S. Geographical distribution and habitat characteristics of Glehnia littoralis Fr. Schmidt in South Korea. Korean Journal of Medicinal Crop Science. 13, 171–177 (2005).
Yang, H., Lu, Q., Wu, B. & Zhang, J. Seed dispersal of east Asian coastal dune plants via seawater–short and long distance dispersal. Flora. 207, 701–706 (2012).
Ma, Y. et al. Conserving plant species with extremely small populations (PSESP) in China. Biodivers Conserv. 22, 803–809 (2013).
Harris, L., Campbell, E. E., Nel, R. & Schoeman, D. Rich diversity, strong endemism, but poor protection: addressing the neglect of sandy beach ecosystems in coastal conservation planning. Divers Distrib. 20, 1120–1135 (2014).
Qian, H. & Klinka, K. Plants of British Columbia: Scientific and common names of vascular plants, bryophytes, and lichens. (UBC Press, 1998).
Zhang, M. & Yang, H. Spatial patterns of Glehnia littoralis populations on sandy seashore and their responses to artificial beach ridge. Chinese Journal of Ecology. 34, 47–52 (2015).
Hiraoka, N. & Oyanagi, M. In vitro propagation of Glehnia littoralis from shoot-tips. Plant Cell Rep. 7, 39–42 (1988).
Hou, Y., Wang, Y., Ren, L., Wang, M. & Ma, D. Effects of nitrogen application amount on the growth and production of Glehnia Littoralis. Journal of Qingdao Agricultural University (Natural Science). 24, 28–30 (2007).
Xu, Y., Gu, X. & Yuan, Z. Lignan and neolignan glycosides from the roots of Glehnia littoralis. Planta Med. 76, 1706–1709 (2010).
Matsuura, H., Saxena, G., Farmer, S. W., Hancock, R. & Towers, G. Antibacterial and antifungal polyine compounds from Glehnia littoralis ssp. leiocarpa. Planta Med. 62, 256–259 (1996).
Almeida, L. P., Vousdoukas, M. V., Ferreira, Ó., Rodrigues, B. A. & Matias, A. Thresholds for storm impacts on an exposed sandy coastal area in southern Portugal. Geomorphology. 143, 3–12 (2012).
Hoggart, S. P. G. et al. The consequences of doing nothing: The effects of seawater flooding on coastal zones. Coast Eng. 87, 169–182 (2014).
Castelle, B. et al. Impact of the winter 2013–2014 series of severe Western Europe storms on a double-barred sandy coast: Beach and dune erosion and megacusp embayments. Geomorphology. 238, 135–148 (2015).
Jansen, P. A., Visser, M. D., Joseph Wright, S., Rutten, G. & Muller Landau, H. C. Negative density dependence of seed dispersal and seedling recruitment in a Neotropical palm. Ecol Lett. 17, 1111–1120 (2014).
Leeuwen, C. H., Sarneel, J. M., Paassen, J., Rip, W. J. & Bakker, E. S. Hydrology, shore morphology and species traits affect seed dispersal, germination and community assembly in shoreline plant communities. J Ecol. 102, 998–1007 (2014).
Larson, J. E., Sheley, R. L., Hardegree, S. P., Doescher, P. S. & James, J. J. Seed and seedling traits affecting critical life stage transitions and recruitment outcomes in dryland grasses. J Appl Ecol. 52, 199–209 (2015).
Ming, J. X. & Hua, Z. G. The characteristics in seed germination and dormancy of four wild species of tree peonies and their bearing on endangerment. Acta Photophysiologica Sinic. 25, 214–221 (1999).
Ishihara, M., Kobayashi, N., Sakamoto, S. & Ishibashi, M. Native habitat, phenology and embryo development of Glehnia littoralis Fr. Schm. in areas surrounding Shimane Peninsula, Japan. Horticultural Research (Japan). 7, 469–473 (2008).
Zhao, Y. Study on seed quality detection and quality standard of Glehnia littoralis Schmidt. Journal of Anhui Agricultural Sciences. 36, 10016–10018 (2008).
Colmer, T. D. & Flowers, T. J. Flooding tolerance in halophytes. New Phytol. 179, 964–974 (2008).
Lowry, D. B., Hall, M. C., Salt, D. E. & Willis, J. H. Genetic and physiological basis of adaptive salt tolerance divergence between coastal and inland Mimulus guttatus. New Phytol. 183, 776–788 (2009).
Xiao, Y., Sun, J., Liu, F. & Xu, T. Effects of salinity and sulphide on seed germination of three coastal plants. Flora. 218, 86–91 (2016).
Isermann, M. & Rooney, P. Biological flora of the British isles: Eryngium maritimum. J Ecol. 102, 789–821 (2014).
Mendoza-González, G., Martínez, M. L. & Lithgow, D. Biological flora of coastal dunes and wetlands: Canavalia rosea (Sw.) DC. J Coastal Res. 30, 697–713 (2014).
Masselink, G., Austin, M., Scott, T., Poate, T. & Russell, P. Role of wave forcing, storms and nao in outer bar dynamics on a high-energy, macro-tidal beach. Geomorphology. 226, 76–93 (2014).
Le, K. An analysis of the recent severe storm surge disaster events in China. Nat Hazards. 21, 215–223 (2000).
Kim, S., Matsumi, Y., Yasuda, T. & Mase, H. Storm surges along the Tottori coasts following a typhoon. Ocean Eng. 91, 133–145 (2014).
Orth, R. J., Luckenbach, M. & Moore, K. A. Seed dispersal in a marine macrophyte: implications for colonization and restoration. Ecology. 75, 1927–1939 (1994).
Catford, J. A. & Jansson, R. Drowned, buried and carried away: effects of plant traits on the distribution of native and alien species in riparian ecosystems. New Phytol. 204, 19–36 (2014).
Yang, G. Historical change and future trends of storm surge disaster in China’s coastal area. Journal of Natural Disasters. 9, 23–30 (2000).
Jiang, J., DeAngelis, D. L., Anderson, G. H. & Smith, T. J. Analysis and simulation of propagule dispersal and salinity intrusion from storm surge on the movement of a marsh–mangrove ecotone in South Florida. Estuar Coast. 37, 24–35 (2013).
Nilsson, C., Brown, R. L., Jansson, R. & Merritt, D. M. The role of hydrochory in structuring riparian and wetland vegetation. Biol Rev. 85, 837–858 (2010).
Farrant, J. M. & Ruelland, E. Plant signalling mechanisms in response to the environment. Environ Exp Bot. 114, 1–3 (2015).
Palacio, S., Lenz, A., Wipf, S., Hoch, G. & Rixen, C. Bud freezing resistance in alpine shrubs across snow depth gradients. Environ Exp Bot. 118, 95–101 (2015).
Rey, P. J. & Alcántara, J. M. Effects of habitat alteration on the effectiveness of plant-avian seed dispersal mutualisms: Consequences for plant regeneration. Perspectives in Plant Ecology Evolution and Systematics. 16, 21–31 (2014).
de Waal, C., Anderson, B. & Ellis, A. G. Dispersal, dormancy and life-history tradeoffs at the individual, population and species levels in southern African Asteraceae. New Phytol. 210, 356–365 (2016).
Wallace, J. M., Held, I. M., Thompson, D. W. J., Trenberth, K. E. & Walsh, J. E. Global Warming and Winter Weather. Science. 343, 729 (2014).
Acknowledgements
The study was supported by the Basic Research Fund of Chinese Academy of Forestry (CAFYBB2016ZD001 and CAFYBB2016SY005) and the Fund of Qingdao Agricultural University.
Author information
Authors and Affiliations
Contributions
H.X.Y. and J.M.C. conceived, wrote and edited the manuscript. H.X.Y. and X.S.L. did the experiments. J.M.C. contributed equally with the first author. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Yang, HX., Chu, JM. & Liu, XS. Natural persistence of the coastal plant Glehnia littoralis along temperate sandy coasts. Sci Rep 7, 42784 (2017). https://doi.org/10.1038/srep42784
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep42784
This article is cited by
-
Comparison of yield and metabolites according to the types of hilling materials utilized during Glehnia littoralis sprout vegetable cultivation
Food Science and Biotechnology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.