Abstract
Risk assessment in patients with acute coronary syndromes (ACS) is critical in order to provide adequate treatment. We performed a systematic meta-analysis to assess the predictive role of serum C-reactive protein (CRP) in patients with ST-segment elevation myocardial infarction (STEMI), treated with primary percutaneous coronary intervention (PPCI). We included 7 studies, out of 1,033 studies, with a total of 6,993 patients with STEMI undergoing PPCI, which were divided in the high or low CRP group, according to the validated cut-off values provided by the corresponding CRP assay. High CRP values were associated with increased in-hospital and follow-up all-cause mortality, in-hospital and follow-up major adverse cardiac events (MACE), and recurrent myocardial infarction (MI). The pre-procedural CRP predicted in-hospital target vessel revascularization (TVR), but was not associated with acute/subacute and follow-up in-stent restenosis (ISR), and follow-up TVR. Thus, pre-procedural serum CRP could be a valuable predictor of global cardiovascular risk, rather than a predictor of stent-related complications in patients with STEMI undergoing PPCI. This biomarker might have the potential to improve the management of these high-risk patients.
Similar content being viewed by others
Introduction
Coronary artery disease (CAD) is the most common cause of death worldwide, with an overall mortality of over 7 million people per year1. Inflammation plays an important, but yet incompletely defined role in CAD and in ACS, particularly by contributing to plaque rupture and erosion, which precedes the formation of the overlying thrombosis2,3. The degree of the thrombus blockage determines the type of the ACS: unstable angina (UA), with partial or intermittent coronary artery occlusion and no myocardial injury; non-ST-elevation myocardial infarction (NSTEMI), with partial or intermittent coronary artery occlusion with myocardial damage, and elevated circulating troponin levels; and STEMI, with complete coronary artery occlusion with myocardial damage, and changes in electrocardiogram4,5. The mortality of STEMI patients is about 12% at 6 months, with higher mortality rates in high-risk individuals. Despite all attempts to improve therapeutic approaches, patients with STEMI continue to have a limited prognosis6,7 and it is important to identify new markers that predict the outcomes in this patient cohort.
CRP is an acute phase reactant produced by hepatocytes in reaction to pro-inflammatory cytokines. Elevated CRP levels have been associated with a decrease in endothelial nitric oxide (NO) production8 and an upregulation in endothelin-1 generation, a potent vasoconstrictor produced by the endothelial cells. This causes endothelial dysfunction, which is the hallmark for arteriosclerosis. Furthermore, the expression of chemokines and adhesion proteins9 is promoted. CRP is considered a risk factor for cardiovascular disease, with the relative risk bordering on those of classical risk factors, such as LDL-cholesterol, arterial hypertension or smoking10,11,12,13,14. Several large population studies have demonstrated that high levels of CRP could be an outcome predictor in patients undergoing elective percutaneous coronary intervention (PCI) for stable coronary artery disease15,16, non-ST-elevation acute coronary syndromes17,18 or mixed populations19,20,21,22,23. However, only few evidences are available regarding the role of CRP as a predictor of outcomes in STEMI patients treated by primary percutaneous coronary intervention (PPCI).
According to the current guidelines, PPCI is the gold standard for the treatment of STEMI patients1,24. PPCI is defined as the PCI in the setting of STEMI, without previous fibrinolysis, and it is indicated in all patients with STEMI in the first 12 hours from symptom onset1. Compared to fibrinolysis, PPCI results in higher rates of infarct-related artery patency, higher rates of myocardial blush and lower rates of complications, such as recurrent ischemia, reinfarction, emergency repeat revascularization procedures, intracranial hemorrhage or death25. After revascularization with PPCI, STEMI patients require a special management. Although the last decades provided tremendous advance in the management of STEMI, the mortality is still high and the management is very expensive. Pre-procedural CRP monitoring could be of use in identifying high-risk patients and guiding the management of the STEMI patients, in order to improve their outcome. We performed a systematic meta-analysis in order to assess the predictive role of serum CRP on in-hospital and follow-up outcomes, in patients with STEMI treated with PPCI.
Results
Study selection
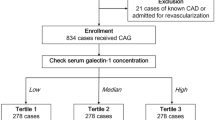
1,033 studies were screened after removing the duplicates from the total amount of papers, 776 irrelevant citations were excluded, 257 full text articles were assessed for eligibility. 46 studies were excluded because they were either reviews, editorials, unrelated meta-analysis, animal studies or subgroup analyses. 204 studies were excluded because they did not meet the inclusion criteria: 2 studies were presented as abstracts, 39 studies did not evaluate PPCI, 109 studies contained mixed populations or other coronary syndromes except for STEMI, 5 studies determined CRP after revascularization or provided no CRP cut-off, 47 studies presented no CRP-outcomes correlation, one study did have a follow-up under 6 months and one study was in Chinese. Consequently, 7 studies were included in our meta-analysis, 6 retrospective studies26,27,28,29,30,31 and 1 prospective cohort study32. The study selection process is shown in Fig. 1. Overall, there were 6,993 patients involved in our analysis, 5,225 included in the low CRP group and 1,768 in the high CRP group. The follow-up period varied between 6 months and 36 months. The characteristics of the selected studies are shown in Table 1. The quality of the included studies was high, with 6 to 8 stars out of a maximum of 9, according to the Newcastle-Ottawa Scale (Table 2). The CRP was assessed by highly sensitive assays methods in all studies, except for Tomoda et al.28. The cut-off value was below 1 mg/dl and defined to be 0.2 mg/dl in one study27, 0.3 mg/dl in three studies26,28,31, 0.5 mg/dl in two studies30,32, and 0.7 mg/dl in one study29.
PRISMA selection flowchart47.
CRP and in-hospital and follow-up all-cause mortality
High CRP was associated with increased in-hospital all-cause mortality, with a RR of 5.62 (95% CI [3.59, 8.78], p < 0.001) assessed from 3 studies26,28,32 reporting this outcome, including 1,222 patients (Fig. 2). The specific causes of death were not described and this is why we called it all-cause mortality. The follow-up all-cause mortality was increased in the high CRP group, with a RR of 2.47 (95% CI [1.78, 3.44], p < 0.001), as obtained from 6 studies26,27,28,29,30,32 which reported this outcome, including 2,721 patients (Fig. 3).
CRP and major adverse cardiac events (MACE)
The in-hospital MACE were increased in the high CRP group, with a RR of 2.91 (95% CI [1.91, 4.42], p < 0.001). The RR was obtained from 4 studies26,28,31,32 which reported this outcome, including 5,492 patients. MACE was defined as a composite of death, target vessel revascularization, recurrent myocardial infarction (MI), and stent reocclusion (Fig. 4). The follow-up MACE RR was 1.68 (95% CI [1.27, 2.22], p < 0.001) after analysing 2,435 patients from 3 studies27,30,31 who reported this outcome (Fig. 5).
CRP and recurrent MI
The recurrent MI risk was increased in the high CRP group, RR was 3.51 (95% CI [1.91, 6.48], p < 0.001) obtained from 4 studies26,27,28,32 which reported this outcome, including 1,480 patients (Fig. 6).
CRP and acute/subacute in-stent restenosis (ISR)
Acute/subacute in-stent restenosis was not different between groups, with RR of 2.01 (95% CI [0.78, 5.2], p = 0.15) derived from analysing 3 studies26,28,32 that reported this outcome, with 1,222 patients (Fig. 7). The follow-up restenosis was not different between the high and low CRP groups, with RR of 1.51 (95% CI [0.76, 3.01], p = 0.24) extracted from 4 studies27,28,30,32 with 929 patients (Fig. 8).
CRP and in-hospital target vessel revascularization (TVR)
In-hospital TVR was increased in the high CRP group, with a RR of 3.16 (95% CI [1.28, 7.76], p = 0.01). To obtain this end-point we analysed data from 3 studies26,28,32 with 1,222 patients. The TVR was defined as coronary arterial by-pass surgery or PCI of the culprit vessel (Fig. 9). The follow-up TVR was similar between the two groups, with an RR of 1.45 (95% CI [0.84, 2.52], p = 0.18) derived from 3 studies27,28,32 which reported this outcome, with 722 patients (Fig. 10).
Heterogeneity between studies, inconsistency and publication bias
There was no significant heterogeneity between studies and the inconsistency was significant in the acute/subacute ISR analysis, where I2 = 61% (Fig. 7). The publication bias was not significant, as assessed by the Egger’s test (Fig. 11).
The sensitivity and subgroup analysis
A sensitivity analysis was performed to address the relative importance of each study, by excluding each study in turn from the analysis. The predictive value of the CRP level maintains for all outcomes. The predictive value of CRP persists when performing the subgroup analysis and comparing the studies with the same CRP cut-off values.
Discussion
This meta-analysis assessed the predictive power of pre-procedural CRP level for short- and long-term outcomes in patients with STEMI treated with PPCI. The study pooled 7 studies, including 6,993 patients.
The main findings of this meta-analysis are:
-
1
Patients with high pre-procedural CRP level have a statistically significant increase in in-hospital and follow-up all-cause mortality, in-hospital and follow-up MACE, and recurrent MI.
-
2
Pre-procedural CRP predicts in-hospital TVR, which is important in the emergency setting, but has no predictive value for the acute/subacute and follow-up ISR, and follow-up TVR.
Many studies assessed the role of CRP in predicting cardiovascular outcomes, but there were no consistent data on the assessment of the CRP predictive value in STEMI patients undergoing PPCI.
The current European Society of Cardiology guidelines do not advise a routinely measurement of CRP, neither in the management of ACS patients1,33, nor in prevention. They indicate that CRP level could improve the risk stratification and could be useful in the management of the statin treatment34. The American Heart Association guideline indicates the measurement of serum CRP to assist risk-based treatment decisions35. Our study findings suggest that CRP might be of tremendous importance in the development of an individual-risk approach in STEMI patients undergoing PPCI.
Our findings are in line with one study20 that assessed the predictive value of CRP in patients undergoing elective PCI and showed that high pre-procedural CRP levels were associated with a higher risk of mortality or MI, but are not related to target vessel revascularization or stent thrombosis. Another study21 on more than 8,800 patients defined CRP as a predictor of all-cause mortality in patients undergoing elective PCI, independent of the LDL cholesterol value. In patients with coronary artery disease undergoing all types of PCI, baseline CRP level predicts one-year mortality and MACE15,22, result which is concordant with our findings. A comprehensive meta-analysis23, including over 34,000 patients that underwent PCI for different conditions, showed that high CRP levels were associated with increased MACE, all-cause mortality, myocardial infarction, coronary revascularization, and clinical restenosis, and concluded that every 1 mg/L in the CRP value was associated with 12% increase in the risk of MACE. There are also studies36 that did not find any association between the risk of stent restenosis after drug-eluting stents (DES) implantation and CRP, which is similar with our findings. On the other side, a meta-analysis37 that included over 2,700 patients undergoing all types of PCI with bare-metal stents (BMS), but not defining subgroups of PPCI, showed that higher baseline CRP levels are associated with higher risk of angiographic restenosis. In the same direction, one study38 showed that patients with CRP < 0.3 mg/dl after follow-up angiography after DES implantation, had a lower risk of MACE and restenosis rate. A meta-analysis39 on 1,062 patients, showed that elevate pre-procedural CRP is associated with greater in-stent restenosis after stenting, with greater impact in unstable-angina patients. So the importance of pre-procedural CRP in predicting stent-related outcomes remains uncertain.
CRP has gained interest as a marker of risk stratification in acute coronary syndromes40, but the most important question would be if this information may influence clinical practice. We have chosen a high-risk group of patients in our meta-analysis, because it is of paramount importance to improve the risk assessment in this group and to tailor the treatment options on the patient’s individual risk. The most important clinically applicable outputs arise from the statin trials, and are based on the pleiotropic anti-inflammatory effect of statins, that reduces the CRP level and consequently improves the prognosis41,42,43,44. Current evidence shows a fundamental role of inflammation in all stages of the atherosclerotic process45,46, but the measures to reduce inflammation have not been yet translated into clinical practice. Thus, our meta-analysis contributes to the potential development of new management protocols of patients with STEMI that undergo PPCI, by selecting, according to the value of CRP, the high risk patients.
Study limitations
Our meta-analysis has some limitation that should be addressed. Firstly, the publication bias may impact the final result, the studies included in the analysis were longitudinal studies, most of them retrospective, and not randomized trial, because there were no randomized controls studies performed regarding our studied population. However, the longitudinal studies reflect the clinic reality and they are useful in decision making. Secondly, the different cut-off values of CRP and the different methods of assessment between studies could be a limitation, as well as the different follow-up times. Thirdly, there was no uniform definition of MACE across the studies.
Conclusion
Pre-procedural serum CRP could be a valuable predictor of the global cardiovascular risk, rather than a predictor of stent-related complications in patients with STEMI undergoing PPCI. This biomarker could help to improve the management of these high-risk patients. The clinical application of determining CRP value before PPCI appears promising, but warrants confirmation by large, well-designed prospective and randomized trials.
Methods
The methods used to perform this work were in compliance with the PRISMA (Preferred Reporting of Items for Systematic Meta-Analysis) statement for studies that evaluate health care interventions47.
Information sources and search strategies
A systematic search of studies published until August 2016 was performed through MEDLINE, Cochrane, EMBASE, and Google Scholar databases, through the major cardiology websites (www.tctmd.com, www.clinicaltrialresult.com, www.medscape.com, www.cardiosource.com), and through the abstracts or presentations of annual meetings of the major cardiovascular societies (European Society of Cardiology and its branches, American Heart Association, American College of Cardiology, Society of Cardiovascular Angiography and Intervention, Transcatheter Cardiovascular Therapeutics, and China Interventional Therapeutics).
We made our search specific and sensitive using the MeSH (Medical Subject Headings) terms (Table 3) and free text. We considered studies in any language. Supplementary Table 1 describes the search result trough Medline performed on the 8th of August 2016.
Inclusion criteria
Studies that fulfilled all the criteria below were included:
-
1
Randomized studies, prospective or retrospective observational design studies.
-
2
Patients with STEMI that undergone PPCI.
-
3
Blood samples for CRP were collected before revascularization and cut-off values for CRP were provided.
-
4
Minimum 6 Months follow-up.
Exclusion criteria
-
1
Subgroup studies, review studies, animal studies, laboratory studies, abstracts.
-
2
Patients that undergone PCI for other pathology (not PPCI) or mixed population without reported outcomes in the PPCI subset.
-
3
Blood samples collected after revascularization.
-
4
No relation between CRP value and clinical outcomes.
Data extraction and quality assessment
Two of the authors (RIM and MT) independently performed data extraction, using a standard data extraction form that contained publication details (name of the first author, year of publication), study design, characteristics of the studied population (sample size, gender distribution), methods of CRP measurement, CRP cut-off, duration of follow-up, and outcomes.
Two of the authors (RIM and MT) assessed independently the trial eligibility, the trail quality, and extracted the data. The trial quality was assessed using the Newcastle-Ottawa Scale48, because the Cochrane Handbook49 risk of bias refers especially to randomised trials. According to this scale, each study is judged on eight items, categorized into three groups: the selection of the study groups, the comparability of the groups, and the ascertainment of either the exposure or outcome of interest for case-control or cohort studies respectively. A maximum of 4 stars for selection, 2 stars for comparability, and 3 stars for outcomes could be awarded. Stars are awarded such that the highest quality studies are awarded up to 9 stars. The guidelines for reporting the meta-analysis of observational studies50 recognizes that the use of quality scoring in meta-analysis of observational studies is controversial and recommends the reporting of quality scoring, if it has been done, and subgroup or sensitivity analysis, rather than using the quality scores.
Study endpoints
The endpoints were: in-hospital and follow-up all-cause mortality, in-hospital and follow-up MACE, recurrent MI, acute or subacute ISR and follow-up ISR, in-hospital and follow-up TVR. MACE were defined as a composite of death, target vessel revascularization, recurrent MI, and stent reocclusion. TVR was defined as coronary arterial by-pass surgery or PCI of the culprit vessel. One study26 reported the outcomes in quartiles of CRP and we considered the first three quartiles as low CRP group, because the CRP value was <0.3 mg/dl and the forth quartile as the high CRP group. In one study27 we considered the total event rate according to the CRP cut-off, irrespective of the stent type. In one study31 we considered the total event rate according to the cut-off value of CRP, without taking into consideration the symptoms-to-balloon time.
Statistical analysis
The meta-analysis was conducted for eligible studies as per risk estimates by two categories: low CRP values and high CRP values. Data are expressed as RR and 95% confidence interval (95% CI) for dichotomous outcomes51. The cut-off value for the high CRP was considered according to the validated cut-off values provided by the corresponding CRP assay. We included in the high CRP group all patients with CRP values above the cut-off provided by the manufacturer of the CRP assay (see Table 1), according to the calibration tests, while the rest of patients were included in the low CRP group. A random-effect, rather than a fixed-effect was adopted, because this is likely the most appropriate and conservative, accounting for differences among trials. Heterogeneity between studies was assessed by Q statistic and inconsistency was quantified with the I2 statistic. Because this test has a poor power in the event of few studies, we considered both the presence of significant heterogeneity at the 10% level of significance and value of I2 ≥56% as an indicator of significant heterogeneity52. The presence of publication bias was assessed by Egger’s test53. All analyses were conducted using Review Manager version 5.3 (Revman, The Cochrane Collaboration, Oxford, United Kingdom).
Additional Information
How to cite this article: Mincu, R.-I. et al. Preprocedural C-Reactive Protein Predicts Outcomes after Primary Percutaneous Coronary Intervention in Patients with ST-elevation Myocardial Infarction a systematic meta-analysis. Sci. Rep. 7, 41530; doi: 10.1038/srep41530 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Steg, P. G. et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 33, 2569–2619 (2012).
Hansson, C. K. Inflammation, atherosclerosis and coronary artery disease. N. Engl. J. Med. 352, 1685–1695 (2005).
Davies, M. J. The pathophysiology of acute coronary syndromes. Heart. 83, 361–366 (2000).
Grech, E. D. & Ramsdale, D. R. Acute coronary syndrome: unstable angina and non-ST segment elevation myocardial infarction. BMJ. 326, 1259–1261 (2003).
Overbaugh, K. J. Acute coronary syndrome. Am. J. Nurs. 109, 42–52 (2009).
Fox, K. A. et al. Underestimated and underrecognized: the late consequences of acute coronary syndrome (GRACE UK– Belgian Study). Eur. Heart J. 31, 2755–2764 (2010).
Marceau, A., Samson, J. M., Laflamme, N. & Rinfret, S. Short and long-term mortality after STEMI versus NON-STEMI: a systematic review and meta-analysis. J. Am. Coll. Cardiol. 61, E96 (2013).
Verma, S. et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation 106, 913–919 (2002).
Verma, S. et al. Endothelin antagonism and interleukin-6 inhibition attenuate the proatherogenic effects of C-reactive protein. Circulation 105, 1890–1896 (2002).
Boekholdt, S. M. et al. C-reactive protein levels and coronary artery disease incidence and mortality in apparently healthy men and women: the EPIC-Norfolk prospective population study 1993–2003. Atherosclerosis 187, 415–422 (2006).
Laaksonen, D. E. et al. C-reactive protein in the prediction of cardiovascular and overall mortality in middle-aged men: a population-based cohort study. Eur. Heart J. 26, 1783–1789 (2005).
Koenig, W. et al. C-reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation 99, 237–242 (1999).
Musunuru, K. et al. The use of high-sensitivity assays for C-reactive protein in clinical practice. Nat. Clin. Pract. Cardiovasc. Med. 5, 621–635 (2008).
Kaptoge, S. et al. C-reactive protein, fibrinogen and cardiovascular disease prediction. N. Engl. J. Med. 367, 1310–1320 (2012).
Ndrepepa, G. et al. Comparative prognostic value of low-density lipoprotein cholesterol and C-reactive protein in patients with stable coronary artery disease treated with percutaneous coronary intervention and chronic statin therapy. Cardiovasc. Revasc. Med. 15, 131–136 (2014).
Nozue, T. et al. C-reactive protein and future cardiovascular events in statin-treated patients with angina pectoris: the extended TRUTH study. J. Atheroscler. Thromb. 20, 717–725 (2013).
Gibson, C. M. et al. Comparison of effects of bare metal versus drug-eluting stent implantation on biomarker levels following percutaneous coronary intervention for non-ST-elevation acute coronary syndrome. Am. J. Cardiol. 15, 1473–1477 (2006).
Nakachi, T. et al. C-reactive protein elevation and rapid angiographic progression of nonculprit lesion in patients with non-ST-segment elevation acute coronary syndrome. Circ. J. 72, 1953–1959 (2008).
Abdi, S. et al. Evaluation of the Clinical and Procedural Predictive Factors of no-Reflow Phenomenon Following Primary Percutaneous Coronary Intervention. Res. Cardiovasc. Med. 4, e25414 (2015).
Delhaye, C. et al. Preprocedural high-sensitivity C-reactive protein predicts death or myocardial infarction but not target vessel revascularization or stent thrombosis after percutaneous coronary intervention. Cardiovasc. Revasc. Med. 10, 144–150 (2009).
Razzouk, L. et al. C-reactive protein predicts long-term mortality independently of low-density lipoprotein cholesterol in patients undergoing percutaneous coronary intervention. Am. Heart J. 158, 277–283 (2009).
Iijima, R. et al. Pre-procedural C-reactive protein levels and clinical outcomes after percutaneous coronary interventions with and without abciximab: pooled analysis of four ISAR trials. Heart 95, 107–112 (2009).
Bibek, S. B. et al. Role of pre-procedural C-reactive protein level in the prediction of major adverse cardiac events in patients undergoing percutaneous coronary intervention: a meta-analysis of longitudinal studies. Inflammation 38, 159–169 (2015).
Windecker, S. et al. 2014 ESC/EACTS Guidelines on myocardial revascularization. Eu. Heart J. 35, 2541–2619 (2014).
Keeley, E. C., Boura, J. A. & Grines, C. L. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 361,13–20 (2003).
Ortolani, P. et al. Predictive value of high sensitivity C-reactive protein in patients with ST-elevation myocardial infarction treated with percutaneous coronary intervention. Eur. Heart J. 29, 1241–1249 (2008).
Schoos, M. M. et al. Usefulness of preprocedure high-sensitivity C-reactive protein to predict death, recurrent myocardial infarction, and stent thrombosis according to stent type in patients with ST-segment elevation myocardial infarction randomized to bare metal or drug-eluting stenting during primary percutaneous coronary intervention. Am. Cardiol. 107, 1597–1603 (2011).
Tomoda, H. & Aoki, N. Prognostic value of C-reactive protein levels within six hours after the onset of acute myocardial infarction. Am. Heart J. 140, 324–328 (2000).
Damman, P. et al. Multiple biomarkers at admission significantly improve the prediction of mortality in patients undergoing primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 57, 29–36 (2011).
Jeong, Y. H. et al. Biomarkers on admission for the prediction of cardiovascular events after primary stenting in patients with ST-elevation myocardial infarction. Clin. Cardiol. 31, 572–579 (2008).
Kim, K. H. et al. The Impact of Ischemic Time on the Predictive Value of High-Sensitivity C-Reactive Protein in ST-Segment Elevation Myocardial Infarction Patients Treated by Primary Percutaneous Coronary Intervention. Korean Circ J. 43, 664–673 (2013).
Magadle, R. et al. The relation between preprocedural C-reactive protein levels and early and late complications in patients with acute myocardial infarction undergoing interventional coronary angioplasty. Clin. Cardiol. 27, 163–168 (2004).
Roffi, M. et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 37, 267–315 (2016).
Catapano, A. L. et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur. Heart J. - Advance Access published August 27 (2016).
Goff, D. C. et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129, S49–S73 (2014).
Park, D. W. et al. Prognostic impact of preprocedural C-reactive protein levels on six-month angiographic and one-year clinical outcomes after drug-eluting stnet implantation. Heart 93, 1087–1092 (2007).
Ferrante, G. et al. Association between C-reactive protein and angiographic restenosis after bare metal stents: an updated and comprehensive meta-analysis of 2747 patients. Cardiovasc. Revasc. Med. 9, 156–165 (2008).
Hsieh, I. C. et al. Prognostic Impact of 9-Month High-Sensitivity C-Reactive Protein Levels on Long-Term Clinical Outcomes and In-Stent Restenosis in Patients at 9 Months after Drug-Eluting Stent Implantation. PLoS One. 10, e0138512 (2015).
Li, J. J. et al. Impact of C reactive protein on in-stent restenosis: a meta-analysis. Tex Heart Inst. J. 37, 49–57 (2010).
Biasucci, L. M. et al. How to use C-reactive protein in acute coronary care. Eu. Heart J. 34, 3687–3690 (2013).
Ridker, P. M. et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 359, 2195–2207 (2008).
Server, P. S. et al. Evaluation of C-reactive protein prior to and on-treatment as a predictor of benefit from atorvastatin: observations from the Anglo-Scandinavian Cardiac Outcomes Trial. Eur. Heart J. 33, 486–494 (2012).
Ridker, P. M. et al. C-reactive protein levels and outcomes after statin therapy. N. Engl. J. Med. 352, 20–28 (2005).
Nissen, S. E. et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N. Engl. J. Med. 352, 29–38 (2005).
Libby, P. Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 32, 2045–51 (2012).
Koenig, W. & Khuseyinova, N. Biomarkers of atherosclerotic plaque instability and rupture. Aterioscler. Thromb. Vasc. Biol. 27, 15–27 (2007).
Liberati, A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann. Intern. Med. 151, W65–94 (2009).
Wells, G. A. et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in metaanalyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (2011).
Higgings, J. & Green, D. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. Cochrane Collaboration. www.cochrane-handbook.org (2011).
Stroup, D. F. et al. Meta-analysis of observational studies in epidemiology, a proposal for reporting. JAMA 283, 2008–2012 (2000).
Viera, A. J. Odds ratios and risk ratios: what’s the difference and why does it matter? South Med. J. 101, 730–734 (2008).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558 (2002).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 315, 629–634 (1997).
Acknowledgements
RIM was supported by a research grant from the European Society of Cardiology (R-2016-013). TR was supported by a grant from the Deutsche Forschungsgemeinschaft (RA 969/4-2).
Author information
Authors and Affiliations
Contributions
R.I.M. and M.T. designed the study; R.I.M. and M.T. performed the literature search and the selection of the studies. R.I.M., R.A.J., D.V., T.R., M.T. interpreted data; R.I.M., T.R. and M.T. wrote the main manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Mincu, RI., Jánosi, R., Vinereanu, D. et al. Preprocedural C-Reactive Protein Predicts Outcomes after Primary Percutaneous Coronary Intervention in Patients with ST-elevation Myocardial Infarction a systematic meta-analysis. Sci Rep 7, 41530 (2017). https://doi.org/10.1038/srep41530
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep41530
This article is cited by
-
Association of peak C-reactive protein with long-term clinical outcomes in patients with ST-segment elevation myocardial infarction
Heart and Vessels (2023)
-
Impact of lactate dehydrogenase on prognosis of patients undergoing cardiac surgery
BMC Cardiovascular Disorders (2022)
-
Assessment of TNF-α expression in unstable atherosclerotic plaques, serum IL-6 and TNF-α levels in patients with acute coronary syndrome and rheumatoid arthritis
Rheumatology International (2022)
-
Inflammation as a determinant of healing response after coronary stent implantation
The International Journal of Cardiovascular Imaging (2021)
-
Global assessment of C-reactive protein and health-related outcomes: an umbrella review of evidence from observational studies and Mendelian randomization studies
European Journal of Epidemiology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.