Abstract
The present study is a summary of the current level of the insecticide resistance to selected organophosphates, pyrethroids, and neonicotinoids in seven Indian field populations of Bemisia tabaci genetic groups Asia-I, Asia-II-1, and Asia-II-7. Susceptibility of these populations was varied with Asia-II-7 being the most susceptible, while Asia-I and Asia-II-1 populations were showing significant resistance to these insecticides. The variability of the LC50 values was 7x for imidacloprid and thiamethoxam, 5x for monocrotophos and 3x for cypermethrin among the Asia-I, while, they were 7x for cypermethrin, 6x for deltamethrin and 5x for imidacloprid within the Asia-II-1 populations. When compared with the most susceptible, PUSA population (Asia-II-7), a substantial increase in resistant ratios was observed in both the populations of Asia-I and Asia-II-1. Comparative analysis during 2010–13 revealed a decline in susceptibility in Asia-I and Asia-II-1 populations of B. tabaci to the tested organophosphate, pyrethroid, and neonicotinoid insecticides. Evidence of potential control failure was detected using probit analysis estimates for cypermethrin, deltamethrin, monocrotophos and imidacloprid. Our results update resistance status of B. tabaci in India. The implications of insecticide resistance management of B. tabaci on Indian subcontinent are discussed.
Similar content being viewed by others
Introduction
The whitefly, Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae), is one of the world’s top 100 invasive organisms1. It is causing severe economic damage in over 60 crop plants as a phloem sap sucking pest or as a vector of viral diseases2. Wider host adaptability, cryptic species status, and virus transmission capabilities have rendered the management of this pest very difficult1. B. tabaci has tremendous potential to develop resistance to insecticides. To date, B. tabaci has shown resistance to more than 40 active ingredients of insecticides3.
Historically, cotton and vegetables have accounted for more than 50 percent of insecticide usage in India4. With the wider adoption of Bt cotton technology in India during 2002, the insecticide usage on cotton for controlling bollworms had started declining5. However, there has been a surge in demand for insecticides on cotton since 2006. As per one estimate, the insecticide usage on cotton in India has increased from 2374 MT in 2006 to 6372 MT in 2011, on account of increase in area under sucking pest susceptible Bt cotton hybrids, resurgence of sucking pests and due to progressive increase in levels of resistance by sucking pests to insecticides4,6,7.
Insecticides have been the mainstay of controlling B. tabaci in diverse agricultural production systems. Organophosphates (OPs) and organochlorine insecticides had been gradually replaced by pyrethroids during the late 70s and 80s8. Subsequently, the OPs and pyrethroids have been replaced by neonicotinoids and other compounds of novel chemistry during the late 90s, worldwide9. Nevertheless, continued use of these compounds for controlling sucking insects such as B. tabaci occurs on the Indian subcontinent7,10. Several field problems such as poor selection of chemicals and sub-standard application practices exacerbated the control failures of insecticides against B. tabaci in India11. The repeated use of compounds of same active ingredients and application of excessive doses of insecticides within a given cropping season has led to the development of insecticide resistance against OPs and pyrethroids in B. tabaci12,13.
Resistance to insecticides resulting in loss of efficacy of many older insecticides has placed excessive pressure on novel products14. Studies have shown the development of resistance in whiteflies to even compounds of novel chemistry in several countries, including Brazil15,16, Burkina Faso17, China18,19, Colombia20, Cyprus21, Egypt22, Germany23,24, Greece25, Guatemala26, India12,27, Iran28, Israel29,30,31,32,33, Italy34, Malaysia35, Nicaragua36, Pakistan37, Spain16, Sudan36, Turkey38, and USA39,40,41,42,43,44,45. India has a long history of resistance to OPs, pyrethroids, and carbamates by bollworms, Helicoverpa armigera (Hübner) and whitefly on cotton12,27,46,47,48. Further, some researchers observed that the preponderance of B. tabaci genetic groups in certain geographical regions had principally been driven by insecticide tolerance levels in specific B. tabaci genetic groups30,49,50. The dominance of B and Q biotypes over indigenous biotypes of B. tabaci especially in China, Israel, North America was largely attributed to their insecticide resistance traits19,31,42,51. Extensive information is available on the insecticide resistance status of Mediterranean (MED) and the Middle East-Asia Minor 1 (MEAM 1) genetic groups, known in older literature as the Q and B biotypes, respectively1. Although Indian geographical regions display an enormous diversity of B. tabaci with the presence of nine out of the 36 genetic groups recorded so far52,53, only limited literature is available on the insecticide resistance status of Indian contingent of B. tabaci species complex10,12,27. The present investigation attempts to take a snapshot view of resistance development in field populations of B. tabaci (collected across agro-climatic zones) against OPs, synthetic pyrethroids and neonicotinoids concurrently used for controlling B. tabaci in India along with information on their genetic group status. Besides, the changes in susceptibility levels of selected B. tabaci field populations against OP, pyrethroid, and neonicotinoid compounds were estimated from 2010 to 2013 for understanding the dynamics of insecticide resistance development in these B. tabaci populations.
Insecticide resistance is often manifested as control failures at field level. Recent studies in Brazil and Greece54,55 explored insecticide resistance of tomato leafminer, Tuta absoluta (Meyrick) deploying analytical tools to estimate the potential control failures. This study attempts to predict potential control failures of the commonly used OP, pyrethroid and neonicotinoid compounds in regional, Indian populations of B. tabaci using probit analysis of existing populations and comparing them to a susceptible population.
Results
Genetic group status of B. tabaci populations
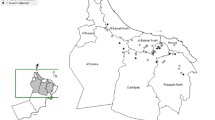
The genetic group status and geographical information of all the B. tabaci populations used in this study are shown in Table 1 and Fig. 1. The mitochondrial cytochrome oxidase 1 sequence analysis showed that each of the B. tabaci populations could be assigned to a single genetic group and it was observed that there was no mixture of different genetic groups in any of the populations. Three B. tabaci populations from Ludhiana, Sriganganagar, and New Delhi were assigned to the Asia-II-1 genetic group, while the populations from Amravati, Khandwa, Guntur, and Nadia belonged to the Asia-I genetic group. The B. tabaci population collected from the cotton fields of the Indian Agricultural Research Institute, Pusa Campus, New Delhi (designated as PUSA population) was assigned to the Asia-II-7 genetic group. The representative sequences of all populations used in this study were deposited in GenBank under accession numbers KF298445 to KF298451, KP641660, and KU613373.
On India map, the states are delimited by thin lines with states in light gray indicate the collection regions. Collection sites are indicated by names and markings; genetic groups of B. tabaci are indicated by different symbols: circle-Asia-1, polygon-Asia-II-1, and square-Asia-II-7. The image was acquired from http://d-maps.com/carte.php?num_car=4183&lang=en; the final image was created using the software Adobe Photoshop Version 7.0 (Adobe Systems, San Jose, CA, USA).
Insecticide usage history and cropping details
The details of Knowledge-Attitude-Practice surveys are presented in Table 1. The surveys were conducted in farmers’ fields in the study locations before the start of this investigation to collect primary data on the cropping and insecticide usage pattern of the farmers in these localities. The surveys revealed that the commercial Bt cotton hybrid seeds available to the farmers had been pre-treated with imidacloprid 70WS; whitefly, B. tabaci, and the leafhopper, Amrasca biguttula biguttula (Ishida) were the major sucking pests on cotton in northern and southern India, while the whiteflies were the predominant sucking pests on brinjal in eastern India. The OPs, pyrethroids, and neonicotinoids were predominantly used by the farmers for control of whitefly in cotton (and brinjal in Nadia) in these regions. The number of spray applications was 10–12 in Nadia (Eastern India), 7–10 in Ludhiana and Sriganganagar locations (Northwestern India), 6–8 in Guntur (Southern India) and 4–6 in Amravati and Khandwa (Central India).
Insecticide bioassays
Insecticide bioassays were conducted in 2013 to generate dose response data for the B. tabaci populations (from different geographic locations) against OP, pyrethroid, and neonicotinoid compounds. The results of dose response regressions analyzed by probit analysis are shown in Table 2. The χ2 analysis showed that dose responses of all the tested populations fitted the log-dose probit mortality model and the linearity was rejected only for cypermethrin against New Delhi and for Nadia populations (Table 2). Resistance ratios were computed separately for Asia-I and Asia-II-1 populations in comparison to the most susceptible B. tabaci population within the respective genetic groups for each insecticide. In the absence of a characterized susceptible strain, we have also computed resistance ratios for the field populations using the PUSA population (Asia-II-7) as the reference check (as it had significantly lower lethal concentration values for all the tested insecticides).
Pyrethroids
The tested B. tabaci populations exhibited the highest slopes in response to the pyrethroids. The slopes of probit response curves ranged from 1.32 to 2.89 for cypermethrin and 1.33 to 4.81 for deltamethrin.
The LC50 values for cypermethrin were in the range of 194 to 1362 mg L−1 among the Asia-I populations, and 238 to 701 mg L−1 among the Asia-II-1 populations. There was upto threefold increase in resistance ratio in Khandwa (Asia-I); four and a sevenfold increase in resistance ratio values respectively in Ludhiana and Sriganganagar (Asia-II-1) in comparison to the most susceptible populations within the respective genetic groups. However, the magnitude of resistance was high in comparison to the PUSA with Sriganganagar and Ludhiana populations recording respectively 136 and 78 fold resistance to cypermethrin, while, the Khandwa population was showing 70 fold resistance to this pyrethroid. The LC50 values for deltamethrin ranged from 120 to 760 mg L−1 in the Asia-II-1 populations and 128 to 242 mg L−1 in the Asia-I populations. Ludhiana and Sriganganagar showed respectively 76 and 71 fold resistance to deltamethrin in comparison to the PUSA (Table 2).
Organophosphates
Triazophos, monocrotophos, and chlorpyrifos were the tested OP compounds. The slopes of the response lines ranged from 1.50 to 2.86 for triazophos; 1.35 to 1.87 for the monocrotophos and 1.57 to 2.34 for chlorpyrifos. For triazophos, the LC50 values ranged from 324 to 525 mg L−1 in Asia-II-1 and 445 to 1429 mg L−1 in Asia-I populations of B. tabaci. A threefold increase in resistance ratio to triazophos was observed in Nadia (Asia-I), while, resistance to triazophos was not significant among the Asia-II-1 populations. The Nadia population was found to be showing 27 fold resistance to triazophos in comparison to the reference PUSA population (Table 2). Analysis of the dose response to monocrotophos showed that the LC50 values were ranging from 528 to 2114 mg L−1 and 843 to 3934 mg L−1 respectively, in the Asia-II-1 and Asia-I populations resulting in fourfold resistance in Ludhiana and fivefold resistance in Nadia in comparison to the susceptible checks within the respective genetic groups. However, the Nadia (Asia-I) and Ludhiana (Asia-II-1) populations recorded significantly higher resistance ratios of 44 and 24 in comparison to the PUSA (Asia-II-7). Among the OP compounds, the chlorpyrifos recorded significantly lower LC50 values of 137 to 201 mg L−1 and 56 to 309 mg L−1 respectively in the Asia-II-1 and Asia-I populations. Comparisons within Asia-I and Asia-II-1 showed a fivefold increase in resistance ratio to chlorpyrifos in Guntur (Asia-I), while no significant increase in resistance ratio was noticed among the Asia-II-1 populations. However, the two Asia-I populations from Guntur and Amravati were showing respectively 25 and 18 fold resistance to chlorpyrifos in comparison to the PUSA population.
Neonicotinoids
Imidacloprid and thiamethoxam were the tested neonicotinoids. The slopes of the response lines to imidacloprid ranged from 1.37 to 2.23 and 1.52 to 2.11 respectively in Asia-I and Asia-II-1 populations. The LC50 values were in the range of 178 to 901 mg L−1 and 130 to 956 mg L−1 respectively, for the Asia-II-1 and Asia-I populations. Sriganganagar and Nadia were showing respectively fivefold and sevenfold resistance to imidacloprid in comparison to the most susceptible population within the respective genetic groups (Table 2). But, these two populations were found to be showing respectively 18 and 17 fold resistance to imidacloprid in comparison to the PUSA population. For thiamethoxam, the LC50 values were ranging from 73 to 194 mg L−1 and 23 to 179 mg L−1 respectively, for the Asia-II-1 and Asia-I populations resulting in upto sevenfold increase in resistance ratio of Guntur (Asia-I) & Amravati (Asia-I) and twofold increase in resistance ratio of Sriganganagar (Asia-II-1) in comparison to the susceptible checks within the respective genetic groups. However, in comparison to PUSA, Sriganganagar, Amravati and Guntur populations showed about a sevenfold increase in resistance ratios to thiamethoxam (Table 2).
Pairwise correlation analysis of LC50
Paired comparisons of the log LC50 values of B. tabaci Asia-II-1 showed positive and significant correlations between cypermethrin and three other insecticides like deltamethrin (r = 0.952, P < 0.1), triazophos (r = 0.988, P < 0.05) and imidacloprid (r = 0.995, P < 0.05). For Asia-II-1, a significant positive correlation was observed between deltamethrin and two other insecticides, monocrotophos (r = 0.994, P < 0.05) and imidacloprid (r = 0.979, P < 0.1). Among the Asia-II-1 populations, significant correlation was observed between triazophos and two other neonicotinoids like imidacloprid (r = 0.967, P < 0.1) and thiamethoxam (r = 0.982, P < 0.1). Further, paired comparisons of the log LC50 values for the insecticides showed positive and significant correlations between triazophos and imidacloprid (r = 0.911, P < 0.05) within the Asia-I populations of B. tabaci. Additionally, a negative correlation was found between chlorpyrifos and most other evaluated insecticides for both the Asia-I and Asia-II-1 populations (Tables 3 and 4).
Control failure likelihood
The analysis of potential control failure likelihood54,55 was done by extrapolation of resistance dataset generated in this investigation (Table 5). The analytical test detected possible cases of control failures for both the pyrethroids for all the field populations of B. tabaci barring PUSA, with the expected mortality (4 to 30% for cypermethrin; <1 to 14% for deltamethrin) at recommended doses were being significantly lower than the lower confidence limits of their estimated LC50 values (Table 5). Similarly, this test detected possible control failure for monocrotophos for all the B. tabaci populations except PUSA with the expected mortality at the recommended field dose (150 mg L−1) being 3 to 21% (Table 5). This test detected possible control failure for triazophos only for the Nadia population of B. tabaci, with its expected mortality being significantly lower than the lower confidence limits of LC50 at the recommended dose (800 mg L−1). No cases of possible control failures were detected for chlorpyrifos against the chosen B. tabaci populations. Regarding the neonicotinoids, possible control failure was detected only for imidacloprid, in all the B. tabaci populations except PUSA with the estimated mortalities (<1 to 22%) at recommended field dose (35.7 mg L−1) were being significantly lower than lower confidence limits of their LC50 estimates. Whereas, for thiamethoxam, this test detected a possible control failure only for Sriganganagar, Ludhiana, Amravati and Guntur populations (Table 5).
Monitoring of resistance in field populations of B. tabaci
The field populations of Guntur, New Delhi, and Sriganganagar were used for monitoring the susceptibility of B. tabaci. The B. tabaci populations were collected from the same fields in three locations during 2010, 2012 and 2013. (The details of collections are summarized in Table 1). The field populations were brought to the laboratory and maintained in separate chambers of insect proof climate control chambers. The mitochondrial cytochrome oxidase 1 sequence analyses revealed that the field populations from the three geographic locations belonged to the same genetic group throughout the course of the investigation with New Delhi and Sriganganagar belonged to Asia-II-1, while, Guntur population was assigned to Asia-I. Changes in dose-mortality responses to imidacloprid, triazophos, and cypermethrin were estimated in the three field populations of B. tabaci during 2010 to 2013 for examining dynamics of resistance (Tables 6, 7 and 8; Figs 2, 3 and 4). Substantial variation in dose responses of these three populations to the selected OP, pyrethroid, and neonicotinoid insecticides during 2010–2013 was noticed. Significant loss in susceptibility to imidacloprid in Guntur population was reflected by the increase in LC50 value from 11 mg L–1 (in 2010) to 130 mg L–1 (in 2013) resulting in the 11 fold rise in the resistance ratio from 2010 to 2013. Substantial variation in response to cypermethrin in this population was revealed by the increase in LC50 values from 25 mg L–1 in 2010 to 261 mg L–1 in 2013. This South Indian B. tabaci population also showed a threefold loss in susceptibility to triazophos during 2010 to 2013 as indicated by the LC50 value of 636 mg L–1 in 2013 compared to the baseline LC50 value of 167 mg L–1 in 2010 (Table 6).
The Mortality response of Guntur population (Asia-I) collected in 2010 to 2013 after the exposure to cypermethrin (a), triazophos (b), and imidacloprid (c). The dose response lines of the each population were drawn using a probit linear model y = αx + β in which α and β are the slope and intercept, respectively. x is the log-transformed dose (mg L−1). y is the percent mortality.
The mortality response of Sriganganagar population (Asia-II-1) collected in 2010 to 2013 after the exposure to cypermethrin (a), triazophos (b), and imidacloprid (c). The dose response lines of the each population were drawn using a probit linear model y = αx + β in which α and β are the slope and intercept, respectively. x is the log-transformed dose (mg L−1). y is the percent mortality.
The mortality response of New Delhi population (Asia-II-1) collected in 2010 to 2013 after the exposure to cypermethrin (a), triazophos (b), and imidacloprid (c). The dose response lines of the each population were drawn using a probit linear model y = αx + β in which α and β are the slope and intercept, respectively. x is the log-transformed dose (mg L−1). y is the percent mortality.
Although the Sriganganagar population was found to be the least susceptible to cypermethrin (LC50 = 1362 mg L–1) and imidacloprid (LC50 = 901 mg L–1) as per dose- response analysis in 2013, there was only threefold rise in the resistance ratio from the baseline susceptibility of these compounds in 2010 (Table 7). The New Delhi population had also shown the substantial loss in susceptibility to cypermethrin from 2010 to 2013 as denoted by the sixfold rise in resistance ratio from 2010 to 2013 and this population also showed about the threefold rise in resistance ratios to triazophos and imidacloprid during 2013 compared to the baseline LC50 estimates generated during 2010 (Table 8).
Discussion
The present study is significant because it gives a summary of the current levels of insecticide resistance expressed by B. tabaci populations belonging to Asia-I, Asia-II-1, and Asia-II-7, drawn across geographical areas of India. Susceptibility of these populations was varied with Asia-II-7 being the most susceptible, while Asia-I and Asia-II-1 populations were showing significant resistance to the selected organophosphate, pyrethroid and neonicotinoid insecticides.
Worldwide, new and novel chemistries have been employed for the control of sucking pests. However, older chemistries are continued to be in use in India, because they are less expensive. The results of our survey in major cotton growing regions in India have also proved this point. The dose response analysis (Table 2) suggests the development of significant resistance to monocrotophos and to a lesser extent to triazophos and chlorpyrifos in Asia-I and Asia-II-1 genetic groups of B. tabaci across geographical areas of India. Compared to an earlier report, there has been a significant increase in the levels of resistance to these OP compounds in the contemporary populations of B. tabaci12. Higher intensity of the insecticides use (frequency, dose, space) leads to genetically based resistances in insects over time56. Very high levels of resistance to monocrotophos noticed in this study in Indian B. tabaci populations, with a magnitude of resistance recorded being higher than ever before, could be attributed to the large scale use of this OP compound by the Indian farmers57. Similarly, high levels of resistance to triazophos noticed in the B. tabaci from Nadia could also be attributed to long term exposure of this B. tabaci population to triazophos. Increased frequency of insecticide usage on vegetable crops has been documented in this region58. Varying levels of resistance to triazophos has earlier been recorded in Asian genetic groups of B. tabaci from India (resistance ratio = 3)10, Pakistan (resistance ratio = 42)37 and in MEAM 1 genetic group of B. tabaci from Turkey (resistance ratio = 310)38. Incipient resistance to chlorpyrifos observed in this study is comparable to the reports on the occurrence of 14 fold resistance to this compound in Asian genetic groups of B. tabaci from Pakistan37.
Low to moderate (resistance ratios ranged from 5 to 45 fold) resistances to cypermethrin have earlier been reported in the B. tabaci populations (which may be belonged to Asia-I considering reports of the predominance of Asia-I in this region53) from southern India12. Our data has shown a considerable increase in the level of resistance to cypermethrin in the contemporary B. tabaci Asia-I and Asia-II-1 populations in India compared to the earlier records12. Especially, Ludhiana and Sriganganagar locations from northern India recorded a high level of resistance to pyrethroids (Table 2). It may be pertinent to note that this region has been an endemic area of cotton leaf curl disease vectored by B. tabaci. We hypothesize that regular outbreaks of cotton leaf curl disease and significantly increased usage of insecticides, including pyrethroids for controlling the vector, could have triggered strong selection pressure for resistance development in these B. tabaci populations. The increased use of pyrethroids was found to be one of the factors linked to the recent outbreak of whitefly in cotton belts of Punjab province of India during 201513. Resistance to pyrethroids has been documented in Asia-I35, MEAM 119,21,24,39,59,60, and MED17,18,19,38,61,62 genetic groups of B. tabaci across the world.
Significant resistance to imidacloprid recorded in this study could be attributed to the long term exposure of this compound in the cotton ecosystem of this country. Since the inception of commercial Bt cotton cultivation in India during 2002, every Bt cotton seed has been mandatory treated with a seed dressing formulation of imidacloprid, besides the application of foliar sprays of imidacloprid by farmers for control of sucking pests including whitefly on cotton7. Consequently, the imidacloprid seed treatment which had earlier conferred protection against sucking pests upto at least 40 to 45 days after sowing (DAS), was later reported to provide protection for only upto 20–25 DAS63. Resistance to neonicotinoids has widely been documented in Asia-I35, MEAM116,18,19,30,41,64,65 and MED16,33,41,42,44,61,64 genetic groups of B. tabaci in many Asian, American, European and Mediterranean countries.
Paired comparisons of the log LC50 values for the insecticides showed significant positive correlations between OP, Pyrethroid and neonicotinoid compounds and a negative correlation was found between chlorpyrifos and other insecticides evaluated in the B. tabaci Asia-I and Asia-II-1 populations (Table 4). In line with the revelation of several earlier works, we speculate the possibility of cross resistance between imidacloprid, OP and pyrethroid compounds. The concurrent occurrence of high levels of resistance to OPs and pyrethroids had been observed in West Africa, Pakistan, and Turkish B. tabaci populations17,37,38. Inconsistency in the neonicotinoid cross-resistance pattern has been reported by Prabhaker et al.41 and by Horowitz et al.30. Earlier reports from china66 and US43 have also demonstrated the prevalence of cross resistance between imidacloprid and thiamethoxam in an MEAM genetic group of B. tabaci34, while, studies with Cyprus populations of B. tabaci (MEAM 1 genetic group) revealed the absence of cross resistance between these two neonicotinoid compounds21,67. Besides target site insensitivity, one or more metabolic resistance mechanisms involving carboxylesterases, cytochrome-P450-dependent monooxygenases, and glutathione S-transferases were implicated in B. tabaci resistant to OP, pyrethroid and neonicotinoid insecticides36,37,38,39,40. It is plausible that Indian B. tabaci populations might have evolved multiple resistance mechanisms in response to field application of these insecticides in the past. Therefore, detailed cross resistance studies need to be undertaken in Indian B. tabaci populations for devising suitable insecticide resistance management strategies.
Several global studies have documented resistance in B. tabaci MED and MEAM 1 genetic groups to different groups of insecticides across the continents3. However, there is a limited literature available on the insecticide status of indigenous B. tabaci genetic groups of Asia. This study clearly provided the insecticide resistance/susceptibility status of Asian genetic groups like Asia-I, Asia-II-1, and Asia-II-7 against the selected OP, pyrethroid, and neonicotinoid compounds. As insecticide resistance is regarded by some workers as a major driving force for the selection and establishment of specific B. tabaci genetic groups in a region19,30,31,51, there is a need for regular monitoring of insecticide resistance status in diverse B. tabaci genetic groups in India.
Knowledge on the susceptibility level of insect populations from different geographical areas is critical for measuring the trends in temporal and spatial resistance development of B. tabaci45. Likewise, our studies have established the decrease in susceptibility levels of three B. tabaci populations to select OP, pyrethroid, neonicotinoid insecticides during 2010 to 2013 (Table 5) and the trend clearly showed the evolution of significant resistances to these insecticides in North Indian field populations of B. tabaci. The recent outbreak of whitefly in the Punjab state of India would appear to be at least partly due to the manifestation of significant resistance development in the field populations of B. tabaci13.
The higher values of LC50 along with the high value of the slopes (Table 2; Figs 2, 3 and 4) may be indicating significant resistance development in Indian field populations of B. tabaci. Chilcuit and Tabashnik68 proposed that slope was not a good indicator of the genetic variability in susceptible organisms, and further, that genetic variation was not related to the LC50 values. However, Hussain et al.69 opined that the higher inter-population variations in the slopes coupled with high level of resistance to the insecticides indicated the possibility of an existence of qualitatively different resistance mechanisms in field strains of H. armigera in Pakistan. Hence, further studies are needed to unravel the biochemical and molecular basis of resistance to these compounds in Indian B. tabaci populations.
The potential for control failure of insecticides was estimated by use of analytical tools as described in Silva et al.54 and Roditakis et al.55. Our results (Table 5) indicate the likelihood of control failures for insecticides such as monocrotophos, imidacloprid, cypermethrin and deltamethrin at the recommended label rates in the selected field populations. Nevertheless, that it was only an estimate and was not based on a rigorous assessment of actual control efficacy of the said chemicals against the field populations. Our results suggest that the field dose of these chemicals have to be higher than the recommended label rate of Central Insecticides Board and Registration Committee, Government of India, to have effective control of B. tabaci at least in these regions. Although insecticide quality is legitimately regulated in India, factors such as poor knowledge on the selection of chemicals by the farmers, use of unscientific tank mixtures and sub-standard application practices exacerbate the problem of control failure of insecticides in field conditions12,63. Hence, appropriate field tests are needed to verify the bioefficacy of these chemicals at recommended label rates against these populations of B. tabaci.
Integrated Pest Management (IPM) has been the overriding principle of plant protection in India and greater emphasis is laid on reducing dependency on chemical control in several crop pests. As our results have shown the widespread development of resistance to OP, pyrethroids and neonicotinoids in the B. tabaci genetic groups, Asia-I and Asia-II-1, we emphasize the need for undertaking regular monitoring of insecticide resistance status of different B. tabaci genetic groups across India. The management of this pest in India may be strengthened by taking clues from successful global IPM programmes.
Host plant resistance is a major, often preventative measure for managing B. tabaci. Studies have shown that pubescent varieties are more preferred by B. tabaci as compared with glabrous ones70,71. Natural defenses in a wild species of cotton, Gossypium arboretum, including long trichome or presence of inorganic salts with increased concentration of waxes provide protection against whitefly and cotton leaf curl virus72. Increasing the area under indigenous varieties of G. arboreum may mitigate the frequent epidemics of whitefly and cotton leaf curl virus especially in Northwestern India.
Rotational scheme of insecticides with different modes of action has been found effective in insecticide resistance management of B. tabaci in Israel. Application of pyriproxyfen in cotton during the first month, followed by an additional treatment with buprofezin (if required), do not markedly alter the susceptibility of B. tabaci to either compounds or no appreciable increase of resistance to the conventional insecticides73. Application of insect growth regulators like pyriproxyfen or buprofezin during the early stage of crop growth is found effective in controlling MEAM 1 genetic group of B. tabaci in Arizona, USA, as these insect growth regulator compounds have helped to conserve natural enemies and substantially reduce sprays of broad-spectrum insecticides74.
The refuge strategy is mandated by the regulatory authorities worldwide to manage the evolution of resistance in bollworms targeted by Bt cotton. Simulation analysis has shown the effectiveness of this strategy in delaying insecticide resistance in MEAM 1 genetic group of B. tabaci75. Although B. tabaci is polyphagous, the cotton refuges have been particularly found more useful in delaying insecticide resistance development in B. tabaci76.
Therefore, a comprehensive, integrated pest management and insecticide resistance management strategies, including identification of whitefly resistant Bt hybrids and G. arboreum genotypes, rotation of conventional insecticides with novel molecules including insect growth regulator (IGR) compounds, use of sticky traps and exploitation of native biological control agents will augur the sustainable management of B. tabaci in the Indian subcontinent.
Methods
Whitefly collection, rearing, and maintenance
The field populations of B. tabaci were collected from seven locations across eastern, central, southern and northern regions of India. Geographically, these locations fall under five agro-climatic zones of India (India has 15 agro-climatic zones). Uniform whitefly infestation pattern and easy accessibility encouraged us to select these regions for the collection of B. tabaci populations. To generate adequate information on the use of insecticide on cotton and vegetable fields, Knowledge-Attitude-Practice (KAP) surveys were conducted in 2010 to 2013 in the study sites by following the protocol used by Yadouleton et al.77. Briefly, ten farmers in each locality were interviewed by using a semi-structured questionnaire focussing on the insecticide application pattern in the farms. Further, qualitative data were collected through direct observations and group discussions. The descriptions of the collection sites, the period of the collections, genetic group identity of B. tabaci populations and the background information on cropping pattern and insecticide application details are presented in Table 1. The exact locations of the collection sites are presented in Fig. 1.
While collecting, standard procedure was followed by walking in ‘Z’ mode at a minimum of two-hectare blocks of the crops. Insects were collected using an aspirator during early morning along with infested leaves containing the nymphs and pupae. The insects were transported to the laboratory in ventilated cages containing leaflets inserted into wet sponges. Infested leaflets were kept in cages for the emergence of fresh adults. The taxonomic identity of B. tabaci species complex was confirmed by examining the insects under a light microscope using the keys of Martin et al.78,79. These populations had been raised on insecticide-free cotton plants (G. hirsutum.) at temperatures of 27 ± 2 °C, photoperiod of 14:10 h (Light:Dark) and relative humidity of 60–70% in quarantined insect growth chambers. These populations were maintained as large colonies for five generations without insecticide selection prior to the current bioassays.
Genetic group determination
The genetic group identity of B. tabaci field population was examined by random sampling of 10 adults for each population using the PCR amplification of mitochondrial cytochrome oxidase 1 gene and sequencing technique as described in Dinsdale et al.80. DNA extraction was performed by using single adult females with the DNeasy Blood and Tissue Kit (Qiagen GmbH, Hilden, Germany). Sequencing was done by outsourcing with SciGenom Labs (Cochin, Kerala, India). Genetic group determination was carried out by the direct sequence comparisons using the web-based Basic Local Alignment Search Tool algorithm of NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The genetic group identity was further confirmed by the phylogenetic and molecular evolutionary analysis with well-assigned homologous sequences of the B. tabaci genetic groups from the consensus sequence database using MEGA version 6 1,81.
Insecticides
Purity analyzed technical grade insecticides such as triazophos (60.9%), monocrotophos (99%) and chlorpyrifos (60%); cypermethrin (99.3%); imidacloprid (96.4%), thiamethoxam (98%) and deltamethrin (98%) were procured from the insecticide manufacturers. These insecticides were selected, as they represented the OPs, pyrethroids and neonicotinoids concurrently used for control of whitefly in the respective regions where the whitefly populations were collected (Table 1). These compounds also had the label claim for efficacy against whiteflies as per the registered use of pesticides with Central Insecticides Board and Registration Committee, Government of India as on date (http://www.cibrc.nic.in).
Bioassay
For assessing the insecticide toxicity to B. tabaci, a modified leaf dip bioassay method of Insecticide Resistance Action Committee was followed82. The stock solutions of technical grade insecticides were prepared in acetone, with serial dilutions in deionized water containing 0.1 g L–1 of non-ionic wetting agent Triton X-100. Cotton leaves with petiole, collected from the fifteen to twenty-five days old seedlings were immersed in the serially diluted insecticide solutions for 20 sec; then allowed to air dry on paper towel and kept on agar slants (2%) in Petri plates (90 × 15 mm). Leaves dipped in only diluents served as the untreated control. The adults were briefly anesthetized using CO2 and transferred in batches of 15–20 onto the treated leaves. The plates were sealed with ventilated lids. All such assays were replicated five times for a minimum of five concentrations for each insecticide. All treatments were placed in an insect rearing room with the temperature, photoperiod, and RH conditions as mentioned earlier. As the mortality rate was too low at earlier hours in some of the doses, observations were taken for an extended period 96 h as described by Gorman et al.16. The adult insect was considered to be dead if no coordinated movement or deficient response to external stimulus (i.e. when gently probed with a fine paintbrush) was observed under the light microscope. Mortality was estimated by counting the total number of dead and live insects.
Monitoring insecticide susceptibility in B. tabaci populations
To compare the changes in susceptibility of B. tabaci populations over time, the populations were collected from same cotton fields located in New Delhi, Sriganganagar, and Guntur (the location details and time of collections are furnished in Table 1). The collection, maintenance and genetic group identity of these B. tabaci field populations were done as described in the earlier section. Dose responses were generated for three insecticides viz., triazophos, cypermethrin, and Imidacloprid during 2010, 2012 and 2013. The details of field populations and their genetic group identities are presented in Table 1.
Data analysis
The mortality data were corrected according to Abbott’s formula83. The LC50 and LC90 values, 95% confidence limits, standard errors, the slopes of the regression lines and χ2 significance tests, were estimated by probit analysis84 using PoloPlus 2.0 software (LeOra Software, California, United States). The resistance ratios were calculated by the “lethal ratio test” and were considered significant when the confidence limits at 95% did not include the value one as proposed by Robertson et al.85. The resistance ratio for each insecticide was calculated with reference to a population of the same genetic group with the lowest LC50 or LC90 17,35. More specifically, resistance ratio = LC50 or LC90 of each population was divided by the LC50 or LC90 of the most susceptible population within Asia-I or Asia-II-1. In the absence of a characterized susceptible strain, the actual resistance level could be underestimated by mere comparisons within the genetic groups. Therefore, additional resistance ratios were also computed for each insecticide with reference to the most susceptible B. tabaci population (belonging to Asia-II-7 collected from New Delhi and designated as PUSA) to demonstrate the magnitude of resistance development in Indian B. tabaci populations in the present dataset.
Pearson correlation coefficient (r) test was applied to test the significance of pairwise comparison between the different attributes (log LC50). The correlation analysis was conducted using SPSS version 16.0. (SPSS Inc. Chicago, Illinois, USA).
The potential for the likelihood of control failure of insecticides was estimated on the basis of Silva et al.54 and Roditakis et al.55. As LC50 is the most reliable point of comparison for dose response regressions85, the 50% mortality was used as a threshold value between control success and failure. The estimated LC50 and 95% confidence limits were compared with the maximum recommended field rate by Central Insecticides Board and Registration Committee, Government of India. The maximum recommended label rates for the tested insecticides in India for whitefly or sucking pest were: cypermethrin 100 mg L−1, deltamethrin 16.67 mg L−1, triazophos 800 mg L−1, monocrotophos 150 mg L−1, chlorpyrifos 250 mg L−1, imidacloprid 35.7 mg L−1 and thiamethoxam 66.67 mg L−1. Briefly, the mortality expressed at the maximum recommended rate was estimated by using the PriProbit Software 1.586. The mortality achieved by the label rate would be considered to be significantly lower than 50% when the lower 95% confidence limits of the LC50 were found to be higher than the recommended rate.
Additional Information
How to cite this article: Naveen, N. C. et al. Insecticide resistance status in the whitefly, Bemisia tabaci genetic groups Asia-I, Asia-II-1 and Asia-II-7 on the Indian subcontinent. Sci. Rep. 7, 40634; doi: 10.1038/srep40634 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
De Barro, P. J., Liu, S.-S., Boykin, L. M. & Dinsdale, A. B. Bemisia tabaci: a statement of species status. Annu. Rev. Entomol. 56, 1–19 (2011).
Navas-Castillo, J., Fiallo-Olivé, E. & Sánchez-Campos, S. Emerging virus diseases transmitted by whiteflies. Annu. Rev. Phytopathol. 49, 219–248 (2011).
Whalon, M. E., Mota-Sanchez, D., Hollingworth, R. M. & Gutierrez, R. Michigan State University, Arthropod Pesticide Resistance Database (2013). Available at: http://www.pesticideresistance.com/Accessed January 5, 2016.
Gutierrez, A. P., Ponti, L., Herren, H. R., Baumgärtner, J. & Kenmore, P. E. Deconstructing Indian cotton: weather, yields, and suicides. Environ. Sci. Eur. 27, 1–17 (2015).
Kranthi, K. R. et al. In-season changes in resistance to insecticides in Helicoverpa armigera (Lepidoptera: Noctuidae) in India. J. Econ. Entomol. 95, 134–142 (2002).
Krishna, V. V. & Qaim, M. Bt cotton and sustainability of pesticide reductions in India. Agric. Syst. 107, 47–55 (2012).
Kranthi, K. R. Bt-Cotton Question and Answer. (Indian society for cotton improvement, 2012).
Casida, J. E. & Quistad, G. B. Golden age of insecticide research: past, present, or future? Annu. Rev. Entomol. 43, 1–16 (1998).
Jeschke, P., Nauen, R., Schindler, M. & Elbert, A. Overview of the status and global strategy for neonicotinoids. J. Agric. Food Chem. 59, 2897–2908 (2010).
Sethi, A. & Dilawari, V. K. Spectrum of insecticide resistance in whitefly from upland cotton in Indian subcontinent. J Entomol 5, 138–147 (2008).
Peshin, R. & Zhang, W. Integrated pest management and pesticide use in Integrated Pest Management (ed. Pimentel, D. & Peshin, R. ) 1–46 (Springer, 2014).
Kranthi, K. R. et al. Insecticide resistance in five major insect pests of cotton in India. Crop Prot. 21, 449–460 (2002).
Kranthi, K. R. Cotton statistics and news http://cicr.org.in/pdf/Kranthi_art/Whitefly.pdf (2015).
Nauen, R. et al. Development of a lateral flow test to detect metabolic resistance in Bemisia tabaci mediated by CYP6CM1, a cytochrome P450 with broad spectrum catalytic efficiency. Pestic. Biochem. Physiol. 121, 3–11 (2015).
Silva, L. D., Omoto, C., Bleicher, E. & Dourado, P. M. Monitoramento da suscetibilidade a inseticidas em populações de Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) no Brasil. Neotrop. Entomol. 38, 116–125 (2009).
Gorman, K. et al. Cross-resistance relationships between neonicotinoids and pymetrozine in Bemisia tabaci (Hemiptera: Aleyrodidae). Pest Manag. Sci. 66, 1186–1190 (2010).
Houndété, T. A. et al. Insecticide resistance in field populations of Bemisia tabaci (Hemiptera: Aleyrodidae) in West Africa. Pest Manag. Sci. 66, 1181–1185 (2010).
Luo, C. et al. Insecticide resistance in Bemisia tabaci biotype Q (Hemiptera: Aleyrodidae) from China. Crop Prot. 29, 429–434 (2010).
Wang, Z., Yan, H., Yang, Y. & Wu, Y. Biotype and insecticide resistance status of the whitefly Bemisia tabaci from China. Pest Manag. Sci. 66, 1360–1366 (2010).
Cardona, C. et al. Resistencia a insecticidas en Bemisia tabaci y Trialeurodes vaporariorum (Homoptera: Aleyrodidae) en Colombia y Ecuador. Rev. Colomb. Entomol. 27, 33–38 (2001).
Vassiliou, V. et al. Insecticide resistance in Bemisia tabaci from Cyprus. Insect Sci. 18, 30–39 (2011).
Kady, H. E. & Devine, G. J. Insecticide resistance in Egyptian populations of the cotton whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). Pest Manag. Sci. 59, 865–871 (2003).
Nauen, R. & Denholm, I. Resistance of insect pests to neonicotinoid insecticides: current status and future prospects. Arch. Insect Biochem. Physiol. 58, 200–215 (2005).
Alon, M. et al. Multiple origins of pyrethroid resistance in sympatric biotypes of Bemisia tabaci (Hemiptera: Aleyrodidae). Insect Biochem. Mol. Biol. 36, 71–79 (2006).
Roditakis, E., Roditakis, N. E. & Tsagkarakou, A. Insecticide resistance in Bemisia tabaci (Homoptera: Aleyrodidae) populations from Crete. Pest Manag. Sci. 61, 577–582 (2005).
Byrne, F. J., Castle, S., Prabhaker, N. & Toscano, N. C. Biochemical study of resistance to imidacloprid in B biotype Bemisia tabaci from Guatemala. Pest Manag. Sci. 59, 347–352 (2003).
Kranthi, K. R., Jadhav, D. R., Wanjari, R. R., Shakir Ali, S. & Russell, D. Carbamate and organophosphate resistance in cotton pests in India, 1995 to 1999. Bull. Entomol. Res. 91, 37–46 (2001).
Basij, M., Talebi, K., Ghadamyari, M., Hosseininaveh, V. & Salami, S. A. Status of Resistance of Bemisia tabaci (Hemiptera: Aleyrodidae) to Neonicotinoids in Iran and Detoxification by Cytochrome P450-Dependent Monooxygenases. Neotrop. Entomol. doi: 10.1007/s13744-016-0437-3 (2016).
Alon, M., Alon, F., Nauen, R. & Morin, S. Organophosphates’ resistance in the B-biotype of Bemisia tabaci Hemiptera: Aleyrodidae) is associated with a point mutation in an ace1-type acetylcholinesterase and overexpression of carboxylesterase. Insect Biochem. Mol. Biol. 38, 940–949 (2008).
Horowitz, A. R., Kontsedalov, S., Khasdan, V. & Ishaaya, I. Biotypes B and Q of Bemisia tabaci and their relevance to neonicotinoid and pyriproxyfen resistance. Arch. Insect Biochem. Physiol. 58, 216–225 (2005).
Horowitz, R., Kontsedalov, S., Khasdan, V., Breslauer, H. & Ishaaya, I. The biotypes B and Q of Bemisia tabaci in Israel-Distribution, resistance to insecticides and implications for pest management. J. Insect Sci. 8, 23–24 (2008).
Kontsedalov, S. et al. Bemisia tabaci Biotype Dynamics and Resistance to Insecticides in Israel During the Years 2008–2010. J. Integr. Agric. 11, 312–320 (2012).
Horowitz, A. R. & Ishaaya, I. Dynamics of biotypes B and Q of the whitefly Bemisia tabaci and its impact on insecticide resistance. Pest Manag. Sci. 70, 1568–1572 (2014).
Nauen, R., Stumpf, N. & Elbert, A. Toxicological and mechanistic studies on neonicotinoid cross resistance in Q-type Bemisia tabaci (Hemiptera: Aleyrodidae). Pest Manag. Sci. 58, 868–875 (2002).
Shadmany, M., Omar, D. & Muhamad, R. Biotype and insecticide resistance status of Bemisia tabaci populations from Peninsular Malaysia. J. Appl. Entomol. 139, 67–75 (2015).
Dittrich, V., Ernst, G. H. & Ruesch, O. Resistance mechanisms in sweet potato whitefly (Homoptera: Aleyrodidae) populations from Sudan, Turkey, Guatemala, and Nicaragua. J. Econ. Entomol. 83, 1665–1670 (1990).
Ahmad, M., Arif, M. I. & Naveed, M. Dynamics of resistance to organophosphate and carbamate insecticides in the cotton whitefly Bemisia tabaci (Hemiptera: Aleyrodidae) from Pakistan. J. Pest Sci. 83, 409–420 (2010).
Erdogan, C., Moores, G. D., Oktay Gurkan, M., Gorman, K. J. & Denholm, I. Insecticide resistance and biotype status of populations of the tobacco whitefly Bemisia tabaci (Hemiptera: Aleyrodidae) from Turkey. Crop Prot. 27, 600–605 (2008).
Cahill, M., Byrne, F. J., Gorman, K., Denholm, I. & Devonshire, A. L. Pyrethroid and organophosphate resistance in the tobacco whitefly Bemisia tabaci (Homoptera: Aleyrodidae). Bull. Entomol. Res. 85, 181–187 (1995).
Denholm, I., Cahill, M., Dennehy, T. J. & Horowitz, A. R. Challenges with managing insecticide resistance in agricultural pests, exemplisfied by the whitefly Bemisia tabaci . Philos. Trans. R. Soc. Lond. B. Biol. Sci. 353, 1757–1767 (1998).
Prabhaker, N., Castle, S., Henneberry, T. J. & Toscano, N. C. Assessment of cross-resistance potential to neonicotinoid insecticides in Bemisia tabaci (Hemiptera: Aleyrodidae). Bull. Entomol. Res. 95, 535–543 (2005).
Dennehy, T. J. et al. Extraordinary resistance to insecticides reveals exotic Q biotype of Bemisia tabaci in the New World. J. Econ. Entomol. 103, 2174–2186 (2010).
Schuster, D. J. et al. Monitoring neonicotinoid resistance in biotype B of Bemisia tabaci in Florida. Pest Manag. Sci. 66, 186–195 (2010).
Castle, S. J. & Prabhaker, N. Monitoring changes in Bemisia tabaci (Hemiptera: Aleyrodidae) susceptibility to neonicotinoid insecticides in Arizona and California. J. Econ. Entomol. 106, 1404–1413 (2013).
Prabhaker, N., Castle, S. & Perring, T. M. Baseline susceptibility of Bemisia tabaci B biotype (Hemiptera: Aleyrodidae) populations from California and Arizona to spirotetramat. J. Econ. Entomol. 107, 773–780 (2014).
Armes, N. J., Jadhav, D. R. & DeSouza, K. R. A survey of insecticide resistance in Helicoverpa armigera in the Indian subcontinent. Bull. Entomol. Res. 86, 499–514 (1996).
Srinivas, R., Udikeri, S. S., Jayalakshmi, S. K. & Sreeramulu, K. Identification of factors responsible for insecticide resistance in Helicoverpa armigera . Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 137, 261–269 (2004).
El-Latif, A. O. A. & Subrahmanyam, B. Pyrethroid resistance and esterase activity in three strains of the cotton bollworm, Helicoverpa armigera (Hübner). Pestic. Biochem. Physiol. 96, 155–159 (2010).
Fernández, E., Grávalos, C., Haro, P. J., Cifuentes, D. & Bielza, P. Insecticide resistance status of Bemisia tabaci Q-biotype in south-eastern Spain. Pest Manag. Sci. 65, 885–891 (2009).
Naranjo, S. E., Castle, S. J., De Barro, P. J. & Liu, S.-S. Population Dynamics, Demography, Dispersal and Spread of Bemisia tabaci in Bemisia: Bionomics and Management of a Global Pest (ed. Stansly P. A. & Naranjo S. E. ) 185–226 (Springer, 2010).
Crowder, D. W. et al. Mating behaviour, life history and adaptation to insecticides determine species exclusion between whiteflies. J. Anim. Ecol. 79, 563–570 (2010).
Hu, J. et al. Global haplotype analysis of the whitefly Bemisia tabaci cryptic species Asia-I in Asia. Mitochondrial DNA 1–10 (2014).
Ellango, R. et al. Distribution of Bemisia tabaci Genetic Groups in India. Environ. Entomol. 44, 1258–1264 (2015).
Silva, G. A. et al. Control failure likelihood and spatial dependence of insecticide resistance in the tomato pinworm, Tuta absoluta . Pest Manag. Sci. 67, 913–920 (2011).
Roditakis, E., Skarmoutsou, C. & Staurakaki, M. Toxicity of insecticides to populations of tomato borer Tuta absoluta (Meyrick) from Greece. Pest Manag. Sci. 69, 834–840 (2013).
Tabashnik, B. E. Managing resistance with multiple pesticide tactics: theory, evidence, and recommendations. J. Econ. Entomol. 82, 1263–1269 (1989).
Ahmad, K. A., Dwivedi, H. S. & Dwivedi, P. Pesticide Scenario of India with particular reference to Madhya Pradesh: A review. 8, 69–76 (2015).
Banerjee, I., Tripathi, S., Roy, As. & Sengupta, P. Pesticide use pattern among farmers in a rural district of West Bengal, India. J. Nat. Sci. Biol. Med. 5, 313 (2014).
Ma, D., Gorman, K., Devine, G., Luo, W. & Denholm, I. The biotype and insecticide-resistance status of whiteflies, Bemisia tabaci (Hemiptera: Aleyrodidae), invading cropping systems in Xinjiang Uygur Autonomous Region, northwestern China. Crop Prot. 26, 612–617 (2007).
Byrne, F. J., Gorman, K. J., Cahill, M., Denholm, I. & Devonshire, A. L. The role of B-type esterases in conferring insecticide resistance in the tobacco whitefly, Bemisia tabaci (Genn). Pest Manag. Sci. 56, 867–874 (2000).
Roditakis, E. et al. Current status of insecticide resistance in Q biotype Bemisia tabaci populations from Crete. Pest Manag. Sci. 65, 313–322 (2009).
Roditakis, E., Tsagkarakou, A. & Vontas, J. Identification of mutations in the para sodium channel of Bemisia tabaci from Crete, associated with resistance to pyrethroids. Pestic. Biochem. Physiol. 85, 161–166 (2006).
Kranthi, K. R. & Russell, D. A. Changing trends in cotton pest management in Integrated pest management: innovation-development process (ed. Peshin, R. & Dhawan, A. ) 499–541 (Springer, 2009).
Karunker, I. et al. Over-expression of cytochrome P450 CYP6CM1 is associated with high resistance to imidacloprid in the B and Q biotypes of Bemisia tabaci Hemiptera: Aleyrodidae). Insect Biochem. Mol. Biol. 38, 634–644 (2008).
Castle, S. J., Merten, P. & Prabhaker, N. Comparative susceptibility of Bemisia tabaci to imidacloprid in field-and laboratory-based bioassays. Pest Manag. Sci. 70, 1538–1546 (2014).
Wang, Z., Yao, M. & Wu, Y. Cross-resistance, inheritance and biochemical mechanisms of imidacloprid resistance in B-biotype Bemisia tabaci . Pest Manag. Sci. 65, 1189–1194 (2009).
Roditakis, E. et al. Assessment of the Bemisia tabaci CYP6CM1vQ transcript and protein levels in laboratory and field-derived imidacloprid-resistant insects and cross-metabolism potential of the recombinant enzyme. Insect Sci. 18, 23–29 (2011).
Chilcuit, C. F. & Tabashnik, B. E. Evolution of Pesticide Resistance and Slope of the Concentration-Mortality Line: Are They Related? J. Econ. Entomol. 88, 11–20 (1995).
Hussain, D., Saleem, H. M., Saleem, M. & Abbas, M. Monitoring of Insecticides Resistance in Field Populations of Helicoverpa armigera (Hub.)(Lepidoptera: Noctuidae). J. Entomol. Zool. Stud. 2, 01–08 (2014).
Chu, C.-C. et al. Susceptibility of upland cotton cultivars to Bemisia tabaci biotype B (Homoptera: Aleyrodidae) in relation to leaf age and trichome density. Ann. Entomol. Soc. Am. 94, 743–749 (2001).
Thomas, A., Naveen, N. C. & Ramamurthy, V. V. An analysis of leaf trichome density and its influence on the morphology of dorsal setae in the puparia of Bemisia tabaci (Hemiptera: Aleyrodidae) on a single cotton leaf. Indian J. Entomol. 76, 128–131 (2014).
Khan, M. A. U. et al. Defense strategies of cotton against whitefly transmitted CLCuV and Begomoviruses. Adv. Life Sci. 2, 58–66 (2015).
Horowitz, A. R., Forer, G. & Ishaaya, I. Managing resistance in Bemisia tabaci in Israel with emphasis on cotton. Pestic. Sci. 42, 113–122 (1994).
Ellsworth, P. C. & Martinez-Carrillo, J. L. IPM for Bemisia tabaci: a case study from North America. Crop Prot. 20, 853–869 (2001).
Crowder, D. W. & Carrière, Y. Comparing the refuge strategy for managing the evolution of insect resistance under different reproductive strategies. J. Theor. Biol. 261, 423–430 (2009).
Carrière, Y. et al. Large-scale, spatially-explicit test of the refuge strategy for delaying insecticide resistance. Proc. Natl. Acad. Sci. 109, 775–780 (2012).
Yadouleton, A. W. M. et al. Development of vegetable farming: a cause of the emergence of insecticide resistance in populations of Anopheles gambiae in urban areas of Benin. Malar. J. 8, 1 (2009).
Martin, J. H. An identification guide to common whitefly pest species of the world (Homopt Aleyrodidae). Int. J. Pest Manag. 33, 298–322 (1987).
Chaubey, R. et al. Morphometric Analysis of Three Putative Species of Bemisia tabaci (Hemiptera: Aleyrodidae) Species Complex from India. Ann. Entomol. Soc. Am. 108, 600–612 (2015).
Dinsdale, A., Cook, L., Riginos, C., Buckley, Y. M. & Barro, P. D. Refined global analysis of Bemisia tabaci (Hemiptera: Sternorrhyncha: Aleyrodoidea: Aleyrodidae) mitochondrial cytochrome oxidase 1 to identify species level genetic boundaries. Ann. Entomol. Soc. Am. 103, 196–208 (2010).
Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. 2013. Mol. Biol. Evol. 30, 2725–2729 (2013).
Naveen, N. C. et al. A model study integrating time dependent mortality in evaluating insecticides against Bemisia tabaci (Hemiptera: Aleyrodidae). Indian J. Entomol. 74, 384–387 (2012).
Abbott, W. S. A method of computing the effectiveness of an insecticide. J Econ Entomol 18, 265–267 (1925).
Finney, D. J. Probit analysis. (Cambridge University Press, Cambridge, UK, 1947).
Robertson, J. L., Russell, R. M., Preisler, H. K. & Savin, N. E. Bioassays with arthropods. (CRC, Boca Raton FL, 2007).
Sakuma, M. Probit analysis of preference data. Appl. Entomol. Zool. 33, 339–348 (1998).
Acknowledgements
The authors would like to thank Pathania, P.C., Nehra, P.L. and Venkateswarulu, P. for field survey support. Furthermore, special thanks are due to Prof. Ramamurthy V.V. and Dr. Shankarganesh for technical support and help rendered during the course of this work. Authors acknowledge support by the insecticide manufactures, National Agricultural Innovation Project (NAIP) and National Agricultural Science Fund (NASF) of the Indian Council for Agricultural Research (ICAR), India. We are thankful to two anonymous reviewers whose corrections and suggestions have helped to improve the quality of the MS by many notches.
Author information
Authors and Affiliations
Contributions
N.C.N. and S.S. conceived and designed experiments. N.C.N. and R.C. contributed to field work. N.C.N. performed the experiment and data analyzed. K.B.R., D.K., R.R. and B.S. contributed reagents/materials/analysis tools/editing of manuscript N.C.N. and S.S. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Naveen, N., Chaubey, R., Kumar, D. et al. Insecticide resistance status in the whitefly, Bemisia tabaci genetic groups Asia-I, Asia-II-1 and Asia-II-7 on the Indian subcontinent. Sci Rep 7, 40634 (2017). https://doi.org/10.1038/srep40634
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep40634
This article is cited by
-
Evidence of population expansion and insecticide resistance mechanism in invasive fall armyworm (Spodoptera frugiperda)
BMC Biotechnology (2023)
-
Bio-pesticides as an ecofriendly management of Whitefly, Bemisia tabaci Mitotype Asia II-1
International Journal of Tropical Insect Science (2023)
-
Changes in defense-related antioxidative enzymes amongst the resistant and susceptible soybean genotypes under whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae) stress
Phytoparasitica (2023)
-
Current knowledge and implementations of Bemisia tabaci genomic technologies for sustainable control
Journal of Pest Science (2023)
-
Integration of insecticidal plant crude protein and the entomopathogenic fungus crude protein against the whitefly, Bemisia tabaci (Homoptera: Aleyrodidae) Mitotype Asia II-1
International Journal of Tropical Insect Science (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.