Abstract
In type 1 diabetes, restoration of normoglycemia can be achieved if the autoimmune attack on beta cells ceases and insulin requirement is met by the residual beta cells. We hypothesize that an adjunctive therapy that reduces insulin demand by increasing insulin sensitivity will improve the efficacy of an immunotherapy in reversing diabetes. We tested the gut microbiota-modulating prebiotic, oligofructose (OFS), as the adjunctive therapy. We treated non-obese diabetic mice with an immunotherapy, monoclonal anti-CD3 antibody (aCD3), with or without concurrent dietary supplement of OFS. After 8 weeks of OFS supplement, the group that received both aCD3 and OFS (aCD3 + OFS) had a higher diabetes remission rate than the group that received aCD3 alone. The aCD3 + OFS group had higher insulin sensitivity accompanied by reduced lymphocytic infiltrate into the pancreatic islets, higher beta-cell proliferation rate, higher pancreatic insulin content, and secreted more insulin in response to glucose. The addition of OFS also caused a change in gut microbiota, with a higher level of Bifidobacterium and lower Clostridium leptum. Hence, our results suggest that OFS can potentially be an effective therapeutic adjunct in the treatment of type 1 diabetes by improving insulin sensitivity and beta-cell function, leading to improved glycemic control.
Similar content being viewed by others
Introduction
Type 1 diabetes (T1D) is an autoimmune disease characterized by the progressive loss of self-tolerance to beta-cell antigens, leading to beta-cell loss1. Several immunotherapeutic approaches have been tested for their ability to halt this process2,3, including the anti-CD3 monoclonal antibody (aCD3). aCD3 has been shown to reverses type 1 diabetes in animal models4,5 and in humans. It slows down beta-cell loss in patients with newly diagnosed T1D by maintaining some endogenous insulin synthesis capacity in the short term6,7,8. Hence, if an adjunct therapy can improve insulin sensitivity thereby reducing insulin requirement, it may improve the therapeutic potential of aCD3 and future immunotherapeutic approaches. A prebiotic may have a role in this context.
Prebiotics are non-digestible plant-derived carbohydrates such as oligofructose (OFS, an inulin-type fructan) and galacto-oligossacharides9,10,11. Fermentation of OFS produces short-chain fatty acids (SCFA) in the colon, which bind to fatty acid receptors and stimulate glucagon-like peptide-1 (GLP-1) and peptide YY, hormones that reduce appetite, delay gastric emptying, and increase insulin sensitivity9,11. In a rodent model of insulin-deficient diabetes, OFS improved glycemia, promoted pancreatic insulin production, and increased beta-cell mass12. OFS may also improve glycemia through its action on gut microbiota9,13,14,15. In both rodent and humans, diabetes is associated with a distinctive gut microbiota16,17,18,19,20,21,22,23, a higher gram-negative to gram-positive bacterial ratio and a lower bifidobacteria abundance. Bifidobacteria is an important microbial population with many health benefits, and treatment with OFS can dose-dependently increase bifidobacteria and improve glucose tolerance24,25. Indeed, bifidobacteria level negatively correlates with fasting insulin, and glucose26.
In this study, our aim was to determine whether OFS given concomitantly with aCD3 can improve the efficacy of aCD3 therapy in inducing diabetes remission in the non-obese diabetic (NOD) mice, which is a widely used rodent model of type 1 diabetes, and to determine the underlying mechanism. Our results show that indeed, addition of OFS as a therapeutic adjunct improved the efficacy of aCD3 therapy, and is associated with higher insulin sensitivity, higher pancreatic insulin content, and higher insulin secretory response during a glucose tolerance test. Furthermore, the group that received OFS had a lower insulitis score and a higher proportion of insulitis-free islets.
Results
The addition of oligofructose increased aCD3-mediated diabetes reversal rate
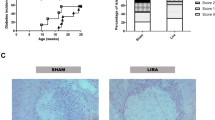
To investigate whether an oligofructose treatment can improve the efficacy of aCD3 immunotherapy in restoring normoglycemia, diabetic NOD mice were treated with aCD3 alone or aCD3 plus OFS (aCD3 + OFS). Treatment response was assessed 8 weeks after the onset of diabetes, at the completion of the 8-week OFS treatment. We found that most of the mice who responded reverted to normoglycemia within the first 2–3 weeks of treatment. A higher proportion of mice in the aCD3 + OFS group achieved normoglycemia (defined as blood glucose <11 mmol/L) in comparison to the aCD3 group (Table 1): at 8 weeks after the onset of diabetes, 9 out of the 12 mice in the aCD3 + OFS group reverted to normoglycemia while only 4 out of the 15 mice in the aCD3 alone group responded (Fisher’s exact test, p = 0.02).
Blood glucose levels were comparable between the two groups at diabetes onset (13.2 ± 0.8 mmol/L for the aCD3 group and 14.0 ± 0.9 mmol/L for the aCD3 + OFS group). Furthermore, there was no difference in blood glucose levels at diagnosis amongst treated mice that achieved diabetes remission and those that did not respond to treatment (responders: 13.2 ± 0.7 mmol/L, non-responders: 13.7 ± 0.9 mmol/L). These results suggest that OFS can improve the rate of aCD3-mediated diabetes remission.
Treatment with oligofructose is associated with improved insulin sensitivity
Restoration of normoglycemia can be achieved by an increase in insulin production capacity and/or a reduction in insulin requirement. To determine which of these mechanisms can account for the higher diabetes remission rate in the aCD3 + OFS group, we assessed glucose homeostasis in mice that were in remission after either treatment regimen. At week 8, we found that the aCD3 + OFS group had lower fasting blood glucose and fasting insulin levels (Table 1). The aCD3 + OFS group had higher insulin sensitivity as measured by homeostasis model assessment of insulin resistance (HOMA-IR) and in response to intraperitoneal injection of insulin during an insulin tolerance test (Fig. 1A). Interestingly, there was no significant difference in glucose excursion during an intraperitoneal glucose tolerance test (IPGTT) (Fig. 1B), although the average blood glucose of the aCD3-alone group tended to be higher than the aCD3 + OFS group throughout the IPGTT. The non-diabetic normal control mice and the aCD3-alone group had similar responses during the IPGTT and ITT, as we previously observed27. Of note, the aCD3-alone and the aCD3 + OFS groups had similar body weights at the time of sacrifice (aCD3: 23.08 ± 0.43 g vs. aCD3 + OFS: 23.35 ± 0.23 g).
Diabetic NOD mice were treated with aCD3 (5 days) ± OFS (8 weeks).
Mice that responded to the treatment and achieved normoglycemia were assessed at the end of the 8-week treatment for insulin sensitivity (A), which was measured by a drop in blood glucose in response to intraperitoneal injection of insulin. Glucose tolerance (B) was measured by IPGTT. Results are presented as mean ± SEM. n = 4–6 mice/treatment group. *p < 0.05 when comparing the aCD3 to the aCD3 + OFS group at the same time point.
The addition of oligofructose resulted in a more robust glucose-stimulated insulin secretion
Next, we determined insulin production capacity by assessing glucose-stimulated insulin secretion and insulin content in mice that responded to treatment and were in diabetes remission. We found that insulin secretion, quantified as integrated area under the curve (AUC) across times 0 to 45 minutes of an IPGTT, was higher in mice treated with aCD3 + OFS in comparison to those treated with aCD3 alone (Fig. 2A). As previously reported and similar to the IPGTT and ITT responses, no difference in insulin secretion was detected between the aCD3-treated group and the non-diabetic control group. Interestingly, several mice in the aCD3 group did not mount a detectable increase in serum insulin level during an IPGTT.
Mice that responded to the treatment and achieved normoglycemia at the end of 8-week treatment were assessed for (A) insulin secretion (measured as integrated area under the curve during the IPGTT). Insulin content (B) was then assessed in pancreas harvested after 8 weeks of treatment. Results are presented as mean ± SEM. n = 4–6 mice/treatment group. *p < 0.05 when comparing the aCD3 to the aCD3 + OFS group at the same time point.
The aCD3 + OFS-treated mice also had higher pancreatic insulin content than the aCD3-treated mice (Fig. 2B). This was accompanied by a higher beta-cell proliferation rate and a trend for higher beta-cell fraction as well as beta-cell mass in the aCD3 + OFS group (Fig. 3). Addition of OFS, however, had no impact on beta-cell size, islet size, or islet numbers per pancreas area. It also had no effect on alpha-cell fraction (i.e. fractional area of pancreas positive for glucagon), number, or size (data not shown).
(A) Beta-cell proliferation, (B) beta-cell fraction, and (C) beta-cell mass were compared between the aCD3 and the aCD3 + OFS group at the en d of the 8-week treatment. (D) Representative images of the islet, immunostained for insulin (red) and BrdU (green). Results are presented as mean ± SEM. n = 4–6 mice/treatment group. *p < 0.05 when comparing the aCD3 to the aCD3 + OFS group.
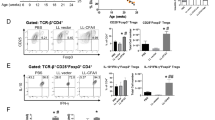
Lymphocytic infiltrate of the pancreatic islets, i.e. insulitis, is a hallmark of immune-mediated beta-cell destruction in NOD mice. Here, we found that the aCD3 + OFS-treated mice had a significantly higher proportion of infiltrate-free islets and a lower total insulitis score than mice treated with aCD3 alone (Fig. 4A-C). We also detected a higher number of Foxp3-positive cells in the pancreatic islets and the pancreatic lymph nodes of the aCD3 + OFS group in comparison to the aCD3-alone group (aCD3: 6.04 ± 1.26% vs. aCD3 + OFS: 14.98 ± 1.53%, p < 0.05; >3500 cells counted for each group)(Fig. 4D).
(A) Insulitis score, (B) percentage of insulitis-free islets, and (C) total insulitis score were compared between the aCD3 and the aCD3 + OFS group at the end of the 8-week treatment. (n = 4–6 mice/treatment group, at least 20 islets were counted from each mouse). (D) Percentage of Foxp3-positive cells in the pancreatic islets and pancreatic lymph nodes. Representative images of pancreatic lymph nodes, immunostained for Foxp3 (red) are shown. (n = 2–3 mice/treatment group, at least 3500 cells were counted for each group).
The composition of gut microbiota changes with oligofructose treatment
We also determined the impact of OFS treatment on the intestinal microbial community, as previous studies in models of type 2 diabetes and obesity suggest that OFS may improve glucose homeostasis by changing the composition of gut microbiota15,28. Here, we found that total bacteria were not affected by OFS. However, there is a significant decrease in Clostridium leptum (p = 0.006) and a significant increase in Bifidobacterium spp. (p = 0.04) in those receiving OFS (Table 1).
Discussion
One of the barriers to successful T1D treatment is the low residual beta-cell mass at the time of diagnosis, which may be insufficient to meet the insulin requirement. The aim of this study was to determine whether a therapeutic adjunct that lowers insulin requirement could improve the efficacy of T1D immunotherapy. We chose OFS because studies have shown that it improves insulin sensitivity in obesity11,15,28,29,30, which may result in lower insulin requirement and improved glucose homeostasis. Our immunotherapy of choice was the anti-CD3 monoclonal antibody (aCD3) because it is a well-characterized agent that induces permanent diabetes remission in up to 60% of NOD mice4,5,27,31, and it has been used in several human trials, demonstrating some efficacy in slowing the process of beta-cell destruction6,7,8,32. In this study, we found that when given in conjunction with aCD3, OFS improves the aCD3-mediated diabetes reversal rate in NOD mice, accompanied by an improvement in insulin sensitivity, a reduction in insulitis, and an increase in beta-cell proliferation rate and insulin secretion.
In a rat model of streptozotocin-induced insulin-deficient diabetes, treatment with OFS improved glucose tolerance, promoted pancreatic insulin production, doubled the beta-cell mass, and up regulated GLP-1 levels, which was assumed to be responsible for the positive effects on the beta-cell12,33. In the NOD mice, we found that OFS improved glucose tolerance, increased pancreatic insulin content, and increased beta-cell proliferation rate which was accompanied by a 2.5-fold increase in beta-cell mass, although the latter did not reach statistical significance. We measured the mRNA expression of proglucagon in the jejunum, the ileum, and the colon. However, we did not detect a difference between the group that received aCD3 + OFS and the group that received aCD3 alone (data not shown). Expression of glucagon in alpha cells is known to be elevated in autoimmune T1D34,35, but whether the expression of proglucagon in the gut is also elevated is unknown. It is possible that our inability to detect a difference in proglucagon expression between the aCD3 + OFS and the aCD3-alone groups is due to the already up regulated proglucagon levels in the diabetic NOD mouse, such that the addition of OFS cannot increase it further. Interestingly, our data suggest that proglucagon mRNA expression is higher in the diabetic NOD mice than those that have not developed diabetes (data not shown).
OFS may improve glycemia through its action on intestinal mucosal barrier function and gut microbiota13,14,15. Patients with T1D and T2D have distinct gut microbiota in comparison to healthy individuals18,19,20,21,23,36, with a higher gram-negative to gram-positive bacterial ratio and a lower bifidobacteria abundance16. Furthermore, diabetes is associated with increased gut permeability, allowing bacterial lipopolysaccharide from the gram-negative bacteria to translocate into the systemic circulation, triggering systemic inflammation and insulin resistance37,38,39,40. OFS treatment dose-dependently increases bifidobacteria24 and improves glucose tolerance in rodents25,26. Indeed, bifidobacteria abundance negatively correlates with fasting insulin and glucose levels in mice with high fat diet-induced diabetes26. OFS also restores tight junction protein expression in the gut mucosa of obese mice38. Here, we found that the OFS-treated NOD mice had a different gut microbiota than those not treated with OFS, with higher levels of Bifidobacterium spp. and lower levels of Clostridium leptum (cluster IV), which are changes that are associated with improved insulin sensitivity. We also determined the expression level of tight junction molecules zonula occludens-1 (ZO-1) and occludin, but did not detect any difference between the treatment groups (data not shown). Interestingly, the group that received OFS had reduced insulitis, which is consistent with the notion that OFS reduces inflammation. This reduction in insulitis likely contributes to the increase in insulin content, as insulitis causes insulin degranulation.
An interesting observation was that although the aCD3 + OFS group secreted more insulin in response to glucose, we did not detect a significant difference in glucose excursion during the IPGTT between the aCD3-alone and the aCD3 + OFS group. One possible explanation is the relatively wide inter-individual variation in glucose response within each treatment group, such that the overall result did not reach statistical significance, although there was a trend for lower glucose levels in the aCD3 + OFS group than the aCD3 group throughout the test (Fig. 2B).
There were some limitations to this study. First, we only used female NOD mice as <30% of male NOD mice develop diabetes, although our finding should also apply to males since the gut dysbiosis observed in T1D and T2D are present in both sexes19,21. Second, in regards to the adverse effects of OFS, some of the mice that received OFS developed a significantly distended abdomen, although they were otherwise healthy with normal blood glucose. This may be unique to the autoimmune T1D model due to the highly inflammatory milieu of these mice. Hence, tolerability of OFS will need to be assessed.
In summary, we demonstrated that the use of an insulin sensitizer, in this case OFS, in conjunction with an immune modulatory therapy (i.e. aCD3), is a viable paradigm to induce diabetes remission in early established diabetes. OFS achieves this goal by improving insulin sensitivity and increasing insulin secretory capacity above a threshold required to maintain normoglycemia. Success of this paradigm ultimately depends on the efficacy of immune therapy to halt beta-cell destruction. Dose-finding studies will need to be performed to find a dose of OFS that has a tolerable gastrointestinal side effect profile but still potent enough to induce the change in gut microbiota required to achieve the desired metabolic effect.
Methods
Mice
Female 6-week old non-obese diabetic (NOD) mice were purchased from Jackson laboratories. Mice were maintained on 12-h light/dark cycles with ad libitum access to food and water. Drinking water was supplemented with 0.8 mg/ml of 5-bromo-2′-deoxyuridine (BrdU) (Sigma-Aldrich) for one week every month to label newly proliferated cells, starting at the onset of diabetes.
Materials
Anti-CD3 monoclonal antibody (mAb145-2C11, anti-mouseCD3) was purchased from BioXCell (West Lebanon, NH, USA). Oligofructose (Orafti® P95) was provided by Beneo GmbH (Mannheim, Germany) and mixed with normal chow (Laboratory rodent diet 5001, Canada Lab Diet, Leduc, Canada) at 1:9 ratio. Mouse ultrasensitive insulin ELISA kit was from ALPCO (Cat# 80-INSMSU-E01; Salem, NH, USA). All chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Ethics
All care and use of animals was approved by the Animal Care Committee at the University of Calgary in accordance with standards of The Canadian Council on Animal Care (Permit number: AC12-0194).
Treatment
Female NOD mice were screened for hyperglycemia twice weekly starting at 12 weeks of age, and diagnosed with diabetes when serum glucose ≥11 mmol/L on 2 consecutive days. Starting on day 1 of diabetes, experimental mice were treated with aCD3 antibody alone (n = 15) or aCD3 plus oligofructose (OFS) (n = 12), while control mice received either OFS only (n = 3) or no active treatment (n = 5). aCD3 was given intravenously at 10 μg/day for 5 consecutive days, a dosing regimen that leads to diabetes remission in up to 2/3 of the animals within 3 weeks4,41. For those receiving both aCD3 and OFS, starting on day 1 of diabetes, 10% OFS (Orafti P95, mixed with normal chow at 1:9 ratio) was provided daily in a food cup for ensuing 8 weeks. We chose an 8-week OFS treatment period because previous work in obese rats showed that 6–8 weeks of OFS was sufficient to induce metabolic benefits and a change in gut microbiota15,27. Blood glucose was monitored twice weekly and diabetes reversal/free was defined as random serum glucose <11 mmol/L for at least 3 consecutive weeks. A group of mice that never developed diabetes were also studied in the same manner as the treated diabetic mice. We were unable to follow the control mice (i.e. mice that received OFS only or no active treatment) long term because they had significant weight loss and became very lethargic, despite of insulin therapy. Consequently, they had to be euthanized ~4 weeks after onset of diabetes.
Tissue Collection
After the mice were sacrificed by cervical dislocation, the entire intestine was excised and fecal matter collected from the cecum and the colon. The full length of the intestine were flushed with PBS and tissue samples were collected from jejunum, ileum, cecum and proximal colon, snap-frozen and stored at −80 °C.
RNA extraction and real-time PCR
Total RNA was extracted from jejunum, ileum, and proximal colon using TRIzol reagent (Invitrogen, Carlsbad, USA). Complementary DNA was synthesized using the Quantitect Reverse Transcription Kit (Qiagen). Reactions were carried out in triplicate with QuantiFast SYBR Green Master Mix (Qiagen) at an annealing temperature of 60 °C. Data were collected using the DNA Engine Opticon2 Continuous Fluorescence Detection System and software (Bio-Rad; Philadelphia, PA, USA). The relative amount of RNA was determined by comparison with phosphoglycerate kinase 1 mRNA as a reference gene. The primer sequences used were as follows: proglucagon – CCAAGATCACTGACAAGAAATAGG (forward), TGTACATCCCAA GTGACTGGC (reverse); zonula occuldens-1 (ZO-1) – GAGTTTCGGGTCCGAGGAG (forward), CATTGCTGTGC TCTTAGCGG (reverse); occludin – TGAACTGTGGATTGGCAG CG (forward), AAGATAAG CGAACCTTGGCG (reverse); and phosphoglycerate kinase 1 (PGK1) – CTCCGCTTTCATGTAGAGGAAG (forward), GACATCTCCTAGTTTGGACAGTG (reverse).
Intraperitoneal glucose tolerance test (IPGTT) and insulin tolerance test (ITT)
IPGTT (glucose at 2 g/kg body weight) and ITT (insulin at 0.5unit/kg body weight) were performed as previously described42. Additional blood samples (~30 μl) were taken at times 0, 15, 30 and 45 minutes of IPGTT for insulin concentration measurements by ELISA.
Pancreatic Insulin Content
Insulin was extracted from the pancreas by incubating the homogenized frozen pancreatic tissue in 1 M HCl/70% ethanol overnight at −20 °C. An equal volume of 1 M TrisHCl (pH = 7.5) was added to the supernatant and stored at −20 °C until insulin determination was carried out by ELISA. Insulin content was normalized to total protein content, which was determined using the BCA Protein Assay Kit (Thermo Scientific, Rockford, Illinois).
Immunofluorescence
Paraffin-embedded pancreas tissue blocks were sectioned longitudinally to 7 μm, and every 40th section was immunostained for insulin and/or glucagon to identify beta cells as described previously42. Nucleus was counter stained by 4′,6-diamidino-2-henylindole (DAPI) to identify cells and to facilitate cell counting. Beta cells were numerated by counting the number of insulin-positive cells by hand. Beta-cell proliferation rate was defined as the percentage of insulin-positive cells that also stained positive for BrdU42. Ten tissue sections, ~2500 cells per mouse, were counted (range 543–5972 cells). Pancreas area was defined by nuclear counter staining. Presence of Foxp3-positive regulatory T cells in pancreatic islets and lymph nodes were detected by immunostaining for Foxp343. Antibodies used included: rat anti-BrdU (Abcam) at 1:100, guinea-pig anti-insulin (Dako), rabbit anti-glucagon (Linco) at 1:500, biotinylated anti-mouse/rat Foxp3 (eBioscience) at 1:100, all diluted in 1% goat serum. Antigen retrieval with Target Retrieval Solution (Dako) or 10 mM sodium citrate buffer was used for Foxp3 or BrdU staining, respectively.
Immunohistochemistry
Paraffin-embedded pancreas sections were immunostained for insulin to identify islets followed by hematoxylin and eosin staining, and degree of lymphocytic infiltration into each islet was scored as following: grade 0 = no infiltrate; grade 1 = peri-islet infiltrate; grade 2 = < 25% of islet area, grade 3 = 25–50% of the islet area, and grade 4 = > 50% of the islet area were infiltrated. At least 20 islets were scored per mouse.
Islet morphometry
Consecutive images of adjacent non-overlapping areas of the entire pancreas section were acquired using a Leica fluorescence microscope or an Olympus FV1000 scanning confocal fluorescence microscope and captured with a CoolSnap digital camera. Images were analyzed by ImageJ software to measure the insulin- or glucagon-positive area as well as the area of the entire pancreas section42. Beta-cell fractions were calculated by dividing the insulin-positive area by the total pancreatic tissue area on the entire section. At least 10 sections were stained and counted for each mouse. Beta-cell mass was determined by multiplying beta-cell fraction by pancreas weight42.
Microbial profiling using qPCR
The contents of the cecum were collected at the time of sacrifice. DNA was extracted from 200 mg cecal matter using the FastDNA Spin kit for feces (MP Biomedicals, LLC, Solon, OH, USA) and quantified using the Nanodrop 2000 (Thermo Fisher Scientific Inc., Waltham, MA, USA). DNA samples were diluted to 4 ng/μl and stored at −20 °C until analysis. Group specific primers, as previously published15, were used to profile select microbial groups using the iCycler (Bio-Rad, Hercules, CA, USA). Purified template DNA from reference strains (ATCC, Manassas, VA, USA) was serially diluted to generate a standard curve. Standard curves were normalized to copy number of 16 S rRNA genes using reference strain genome size and 16 S rRNA gene copy number values obtained from the rrnDB44. The specificity of the primers and the limit of detection were determined according to Louie et al.45 Threshold cycle values were used to calculate the number of 16 S rRNA gene copies in each sample.
Statistical analysis
All statistics were performed using GraphPad Prism VI software (San Diego, CA, USA). Results are expressed as mean ± SEM and comparisons made between the CD3 and CD3 + OFS groups unless otherwise specified. Data was analyzed by two-tailed Student’s t-test. Proportions of diabetic mice that reverted to normoglycemia were compared by Fisher’s test. The minimum level of statistical significance was set at p < 0.05.
Additional Information
How to cite this article: Chan, C. et al. Oligofructose as an adjunct in treatment of diabetes in NOD mice. Sci. Rep. 6, 37627; doi: 10.1038/srep37627 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Tsai, S., Shameli, A. & Santamaria, P. CD8+ T cells in type 1 diabetes. Adv Immunol 100, 79–124 (2008).
Santamaria, P. The long and winding road to understanding and conquering type 1 diabetes. Immunity 32, 437–45 (2010).
Clemente-Casares, X., Tsai, S., Huang, C. & Santamaria, P. Antigen-specific therapeutic approaches in Type 1 diabetes. Cold Spring Harb Perspect Med 2, a007773 (2012).
Chatenoud, L., Thervet, E., Primo, J. & Bach, J. F. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci USA 91, 123–7 (1994).
Chatenoud, L., Primo, J. & Bach, J. F. CD3 antibody-induced dominant self tolerance in overtly diabetic NOD mice. J Immunol 158, 2947–54 (1997).
Herold, K. C. et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 346, 1692–8 (2002).
Keymeulen, B. et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 352, 2598–608 (2005).
Sherry, N. et al. Teplizumab for treatment of type 1 diabetes (Protege study): 1-year results from a randomised, placebo-controlled trial. Lancet 378, 487–97 (2011).
Delzenne, N. M., Cani, P. D. & Neyrinck, A. M. Modulation of glucagon-like peptide 1 and energy metabolism by inulin and oligofructose: experimental data. J Nutr 137, 2547S–2551S (2007).
Nakamura, Y. K. & Omaye, S. T. Metabolic diseases and pro- and prebiotics: Mechanistic insights. Nutr Metab (Lond) 9, 60 (2012).
Kellow, N. J., Coughlan, M. T. & Reid, C. M. Metabolic benefits of dietary prebiotics in human subjects: a systematic review of randomised controlled trials. Br J Nutr 111, 1147–61 (2014).
Cani, P. D. et al. Involvement of endogenous glucagon-like peptide-1(7-36) amide on glycaemia-lowering effect of oligofructose in streptozotocin-treated rats. J Endocrinol 185, 457–65 (2005).
Roberfroid, M. et al. Prebiotic effects: metabolic and health benefits. Br J Nutr 104 Suppl 2, S1–63 (2010).
Geurts, L., Neyrinck, A. M., Delzenne, N. M., Knauf, C. & Cani, P. D. Gut microbiota controls adipose tissue expansion, gut barrier and glucose metabolism: novel insights into molecular targets and interventions using prebiotics. Benef Microbes 5, 3–17 (2014).
Bomhof, M. R., Saha, D. C., Reid, D. T., Paul, H. A. & Reimer, R. A. Combined effects of oligofructose and Bifidobacterium animalis on gut microbiota and glycemia in obese rats. Obesity (Silver Spring) 22, 763–71 (2014).
Picard, C. et al. Review article: bifidobacteria as probiotic agents – physiological effects and clinical benefits. Aliment Pharmacol Ther 22, 495–512 (2005).
Brugman, S. et al. Antibiotic treatment partially protects against type 1 diabetes in the Bio-Breeding diabetes-prone rat. Is the gut flora involved in the development of type 1 diabetes? Diabetologia 49, 2105–8 (2006).
Vaarala, O. Gut microbiota and type 1 diabetes. Rev Diabet Stud 9, 251–9 (2012).
Qin, J. et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490, 55–60 (2012).
Serino, M. et al. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut 61, 543–53 (2012).
de Goffau, M. C. et al. Fecal microbiota composition differs between children with beta-cell autoimmunity and those without. Diabetes 62, 1238–44 (2013).
Ellekilde, M. et al. Characterization of the gut microbiota in leptin deficient obese mice – Correlation to inflammatory and diabetic parameters. Res Vet Sci 96, 241–50 (2014).
Hansen, A. K., Hansen, C. H., Krych, L. & Nielsen, D. S. Impact of the gut microbiota on rodent models of human disease. World J Gastroenterol 20, 17727–36 (2014).
Parnell, J. A. & Reimer, R. A. Prebiotic fibres dose-dependently increase satiety hormones and alter Bacteroidetes and Firmicutes in lean and obese JCR:LA-cp rats. Br J Nutr 107, 601–13 (2011).
Everard, A. et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 60, 2775–86 (2011).
Cani, P. D. et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 50, 2374–83 (2007).
Hyslop, C. M., Tsai, S., Shrivastava, V., Santamaria, P. & Huang, C. Prolactin as an Adjunct for Type 1 Diabetes Immunotherapy. Endocrinology 157, 150–65 (2016).
Bomhof, M. R., Paul, H. A., Geuking, M. B., Eller, L. K. & Reimer, R. A. Improvement in adiposity with oligofructose is modified by antibiotics in obese rats. Faseb J 30, 2720–32 (2016).
Cani, P. D. et al. Improvement of glucose tolerance and hepatic insulin sensitivity by oligofructose requires a functional glucagon-like peptide 1 receptor. Diabetes 55, 1484–90 (2006).
Gao, Z. et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 58, 1509–17 (2009).
Clemente-Casares, X. et al. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature (2016).
Chatenoud, L. Immune therapy for type 1 diabetes mellitus-what is unique about anti-CD3 antibodies? Nat Rev Endocrinol 6, 149–57 (2010).
Drucker, D. J. The role of gut hormones in glucose homeostasis. J Clin Invest 117, 24–32 (2007).
Wang, M. Y. et al. Glucagon receptor antibody completely suppresses type 1 diabetes phenotype without insulin by disrupting a novel diabetogenic pathway. Proc Natl Acad Sci USA 112, 2503–8 (2015).
Yip, L., Taylor, C., Whiting, C. C. & Fathman, C. G. Diminished adenosine A1 receptor expression in pancreatic alpha-cells may contribute to the pathology of type 1 diabetes. Diabetes 62, 4208–19 (2013).
Hansen, A. K., Krych, L., Nielsen, D. S. & Hansen, C. H. A Review of Applied Aspects of Dealing with Gut Microbiota Impact on Rodent Models. Ilar J 56, 250–64 (2015).
Cani, P. D. et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–72 (2007).
Cani, P. D. et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58, 1091–103 (2009).
Lassenius, M. I. et al. Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes Care 34, 1809–15 (2011).
Boerner, B. P. & Sarvetnick, N. E. Type 1 diabetes: role of intestinal microbiome in humans and mice. Ann N Y Acad Sci 1243, 103–18 (2011).
Sherry, N. A. et al. Exendin-4 improves reversal of diabetes in NOD mice treated with anti-CD3 monoclonal antibody by enhancing recovery of beta-cells. Endocrinology 148, 5136–44 (2007).
Huang, C., Snider, F. & Cross, J. C. Prolactin receptor is required for normal glucose homeostasis and modulation of beta-cell mass during pregnancy. Endocrinology 150, 1618–26 (2009).
Maruyama, T. et al. Distribution of Th17 cells and FoxP3(+) regulatory T cells in tumor-infiltrating lymphocytes, tumor-draining lymph nodes and peripheral blood lymphocytes in patients with gastric cancer. Cancer Sci 101, 1947–54 (2010).
Stoddard, S. F., Smith, B. J., Hein, R., Roller, B. R. & Schmidt, T. M. rrnDB: improved tools for interpreting rRNA gene abundance in bacteria and archaea and a new foundation for future development. Nucleic Acids Res 43, D593–8 (2015).
Louie, T. J. et al. Differences of the Fecal Microflora With Clostridium difficile Therapies. Clin Infect Dis 60 Suppl 2, S91–7 (2015).
Acknowledgements
We thank Kristine Lee and Ana Cruz for technical assistance. Clement Chan was supported by a summer studentship from Alberta Innovates Health Solution. Vipul Shrivastava was supported by a CIHR training grant. Andrea Ochoa was supported by a studentship from Globalink. The work was supported by grants from Canadian Diabetes Association and Alberta Children’s Hospital Foundation (to CH) and from Canadian Institutes of Health Research (to RR MOP 115076-1).
Author information
Authors and Affiliations
Contributions
C.C. performed the experiments and edited the manuscript. C.M.H., V.S., and A.O. performed the experiments and reviewed the manuscript. R.A.R. and C.H. conceived and designed the study, and wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Chan, C., Hyslop, C., Shrivastava, V. et al. Oligofructose as an adjunct in treatment of diabetes in NOD mice. Sci Rep 6, 37627 (2016). https://doi.org/10.1038/srep37627
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep37627
This article is cited by
-
Potential Glioprotective Strategies Against Diabetes-Induced Brain Toxicity
Neurotoxicity Research (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.