Abstract
Previous studies have reported controversial results on the association between tomato consumption and prostate cancer risk. Hence, we performed a meta-analysis to comprehensively evaluate this relationship. A total of 24 published studies with 15,099 cases were included. Relative risks (RR) and 95% confidence intervals (CI) were pooled with a random-effects model. Tomato intake was associated with a reduced risk of prostate cancer (RR 0.86, 95% CI 0.75–0.98, P = 0.019; P < 0.001 for heterogeneity, I2 = 72.7%). When stratified by study design, the RRs for case-control and cohort studies were 0.76 (95% CI 0.61–0.94, P = 0.010) and 0.96 (95% CI 0.84–1.10, P = 0.579), respectively. In the subgroup analysis by geographical region, significant protective effects were observed in Asian (RR 0.43, 95% CI 0.22–0.85, P = 0.015) and Oceania populations (RR 0.81, 95% CI 0.67–0.99, P = 0.035), but not in other geographical populations. Begg’s test indicated a significant publication bias (P = 0.015). Overall, tomato intake may have a weak protective effect against prostate cancer. Because of the huge heterogeneity and null results in cohort studies, further prospective studies are needed to explore the potential relationship between tomato consumption and prostate cancer risk.
Similar content being viewed by others
Introduction
Emerging evidence from epidemiological, as well as cell culture and animal, studies indicates that lycopene and the consumption of lycopene-containing foods may be protective against cancer and cardiovascular disease risk1, notably stroke2, hypertension3, and prostate cancer4,5.
Processed tomato products are the primary dietary lycopene source6. The association between tomato food and prostate cancer has been investigated by numerous epidemiological studies, with inconsistent results. Some reported that individuals with higher intake of tomato foods had a lower risk of prostate cancer compared with consumers of lower tomato intake7,8,9,10,11,12,13,14,15, while others found null results16,17,18,19,20. Darlington et al.21 even reported a positive association between consumption of tomato and incidence of prostate cancer.
A previous meta-analysis published in 2004 reported that tomato consumption might play a protective role in the prevention of prostate cancer based on three cohort and seven case-control studies22. However, a latest meta-analysis of seven cohort studies from the World Cancer Research Fund (2014) failed to confirm this association23. The overall purpose of the present study was to evaluate the strength of this controversial association, by performing a systematic review and meta-analysis of all eligible cohort and case-control studies published on the subject in peer-reviewed literature up to now. In addition, we performed a stratified analysis by geographical region to explore the potential regional differences.
Results
Literature search and study characteristics
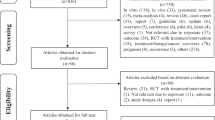
Figure 1 presents the detailed process of literature review. A total of 24 eligible studies7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,24,25,26,27,28,29,30,31,32 were eventually included in this meta-analysis aimed to comprehensively evaluate the relationship between tomato intake and prostate cancer risk. There were 7 cohort and 17 case-control studies, which were performed in the following geographical regions: Europe (n = 4), North America (n = 10), Asia (n = 7), and Oceania (n = 3). Up to 15,099 cases were analyzed in these studies published between 1989 and 2016. Data on exposure (tomato intake) was mainly collected by interview or questionnaire and outcome (prostate cancer) was confirmed histologically in the majority of the included studies. The study quality was evaluated by the Newcastle-Ottawa Scale (NOS). Scores ranged from 5 to 8, with a mean of 6.08. Table 1 summaries the main characteristics of all included studies analyzed in this meta-analysis.
Pooled analysis and heterogeneity assessment
Multivariable adjusted relative risks (RRs) with their confidence intervals (CIs) for each individual study and for the combination of all included studies are shown in Fig. 2. In a random-effect pooled analysis of these studies, high-tomato intake (comparing the highest with the lowest category) was associated with a reduced prostate cancer risk (RR 0.86, 95% CI 0.75–0.98, P = 0.019). Statistically significant heterogeneity was observed among included studies (P < 0.001 for heterogeneity, I2 = 72.7%).
Subgroup analysis
The effects of tomato intake on prostate cancer risk in subgroup meta-analyses are shown in Table 2. We firstly performed stratified analyses by geographical region, significant protective effects of tomato intake against prostate cancer were observed in Asian (RR 0.43, 95% CI 0.22–0.85, P = 0.015) and Oceania populations (RR 0.81, 95% CI 0.67–0.99, P = 0.035), but the effects were not significant in other geographical populations. When stratified by study design, the analysis of case-control studies yielded a RR of 0.76 (95% CI 0.61–0.94, P = 0.010), whereas the analysis based on cohort studies yielded a RR of 0.96 (95% CI 0.84–1.10, P = 0.579) (Fig. 3). In the subgroup analysis by study quality, more pronounced association was detected in studies with low quality (RR 0.77, 95% CI 0.61–0.98, P = 0.030) compared with high-quality studies (RR 0.92, 95% CI 0.79–1.06, P = 0.234). Finally, in the stratified analyses by sample size, statistically significant association was observed in those small studies (RR 0.69, 95% CI 0.54–0.89, P = 0.005) rather than in large studies (RR 0.98, 95% CI 0.86–1.12, P = 0.763).
Sensitivity analysis and publication bias
The influence of each study on the pooled RR was evaluated by repeating the overall analysis after omitting each study in turn. The results indicated that no single study dominated the combined RR. The 24 study-specific RRs ranged from a low of 0.83 (95% CI 0.72–0.97) to a high of 0.89 (95% CI 0.79–1.00) via omission of the study by Stram et al.20 and the study by Jian et al.11, respectively (Fig. 4). Finally, significant publication bias was observed in Begg’s test (P = 0.015), but not in Egger’s test (P = 0.122).
Discussion
This systematic review and meta-analysis aimed to evaluate the relationship between tomato intake and prostate cancer risk based on 7 cohort studies and 17 case-control studies, with a total of 15,099 cases. The results of this quantitative meta-analysis provided limited evidence for a protective effect of high tomato food consumption for prostate cancer incidence. Although the overall analysis suggested a moderate reduction in risk, the results from the cohort, high-quality, and large studies were null.
The findings of this meta-analysis are basically consistent with a previous meta-analysis published in 200422, which included three cohort and seven case-control studies. Its results also indicated that tomato consumption might play a protective role in the prevention of prostate cancer. But the effect was modest and restricted to high amounts of tomato intake22. Since then, emerging studies on this topic have been published, while the results were still conflict. In 2014, a meta-analysis of seven cohort studies from the World Cancer Research Fund reported no significant association between tomato intake and prostate cancer risk. The combined RR per 1 serving/day was 0.93 (95% CI 0.79–1.09; I2 = 52.0%)23. Similarly, when stratified by study design in this study, the analysis based on cohort studies yielded a RR of 0.96 (95% CI 0.84–1.10, I2 = 54.1). Therefore, a protective effect of tomato intake on the risk of prostate cancer is mainly observed in case-control studies. Compared with these previous meta-analyses, the present updated meta-analysis also performed a stratified analysis by geographical region, which provided a more comprehensive assessment of the association between tomato consumption and prostate cancer risk.
Several potential mechanisms could explain the potential cancer-protective effects of tomato food. Tomato food has high levels of lycopene, which has been shown to inhibit prostate cancer progression in several studies. Yang et al.33 reported that lycopene could suppress the proliferation of androgen-dependent human prostate tumor cells (LNCaP) through activation of PPARγ-LXRα-ABCA1 pathway. Elgass et al.34 found that lycopene could also inhibit the cell adhesion and migration properties in androgen-independent prostate cancer cells (PC3 and DU145). In vivo studies, dietary tomato and lycopene could have an influence on androgen signaling- and carcinogenesis-related gene expression during early transgenic adenocarcinoma of the mouse prostate (TRAMP) mice prostate carcinogenesis35. In epidemiological studies, lycopene consumption (both dietary intake and its blood levels) has been linked to a reduced risk of prostate cancer4.
This study had several important strengths. First, as individual studies may have limited statistical power, our meta-analysis of 24 published studies with 15,099 prostate cancer cases might provide more reliable results with greater precision and power. Second, we extracted data from the most fully adjusted model in each study, which reduce the potential influence of confounding factors. Third, various subgroup analyses, influence analysis, and publication bias analysis were performed to evaluate the robustness of the pooled risk estimate.
However, several limitations should be considered in interpreting the results of this meta-analysis. First, there was substantial heterogeneity across studies (P < 0.001 for heterogeneity, I2 = 72.7%), which was likely due to the variation in population information, exposure definitions, exposure ranges, exposure and outcome assessment methods between studies. Second, Begg’s test suggested the existence of publication bias. Although we adopted a loose search strategy, some inevitable publication bias might exist as small studies with negative results were less likely to be published and gray literature (such as non-English articles and conference abstract) was difficult to find. Third, the cutoff points for the lowest and highest categories of the tomato intake were various in included studies, which might also has an influence on the combined risk estimate. Finally, the association between lifestyle factors and prostate cancer risk may vary by tumor characteristics (e.g., stage and grade). However, most of the included studies didn’t provide risk estimates for localized/low grade and advanced/high grade cancers separately. Therefore, we were not able to examine if there were differences by stage and grade in the association between tomato intake and prostate cancer risk.
Conclusion
In summary, this meta-analysis indicates that tomato intake may be associated with a reduced risk of prostate cancer. The significant protective effects were observed in Asian and Oceania populations, but not in other geographical populations. As there were no significant results in cohort and high-quality studies, no firm conclusions can be drawn at the present time. Further large-scale prospective cohorts, as well as mechanistic studies, are needed to clarify the relationship between tomato food intake and prostate cancer risk.
Materials and Methods
Literature review
A comprehensively literature search of published articles was performed in June 2016 based on PubMed and Web of Science databases. We found that few studies were eligible when only using “tomato” and “prostate cancer” as search terms. Therefore, we adopted the following loose search algorithm: (“diet” or “nutrition” or “vegetable” or “vegetables” or “tomato” or “tomatoes” or “lycopene”) and (“prostatic neoplasms” or “prostatic cancer” or “prostate neoplasms” or “prostate cancer”). Furthermore, the cited references of retrieved articles and reviews were also checked to identify any additional relevant studies. There was no language, publication date, or publication status restrictions. This systematic review and meta-analysis was designed, performed, and reported in accordance with the standards of quality for reporting meta-analyses, except for not publishing the review protocol in advance36.
Study selection criteria
A study was included if it met the following criteria: (i) the exposure of interest was consumption of tomato food; (ii) the outcome of interest was incidence of prostate cancer; (iii) study design was cohort, nested case-control or case-control; and (iv) the effect sizes with their corresponding 95% CIs were reported. If multiple articles reported data based on the same population, the publication with the most up-to-date or comprehensive information was included in the meta-analysis.
Study quality assessment
A 9-star system on the basis of the NOS (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp) was used to assess the quality of each included study by two independent reviewers (XX and JFL). NOS judges a study according to the following three broad perspectives: selection (four items), comparability (one item), and exposure/outcome (three items). Each item is awarded one point, except for comparability (two points). Hence, the full score is 9 stars. A study with ≥7 awarded stars is classified as high quality.
Data extraction
Information was collected and recorded independently by two investigators (XX and JFL). Any discrepancies were resolved through iteration and consensus. The following data were obtained from each study: first author’s surname, country, publication year, study design, age, number of cases, instrument of exposure measurement, method of outcome assessment, results of studies (adjusted risk estimates with their corresponding 95% CIs), and matched or adjusted confounding factors in the design or statistical analysis.
Statistical methods
Considering that prostate cancer is a rare disease, the odds ratio (OR) was assumed approximately the same as RR, and the RR was designated as the study outcome. Multiple adjusted RRs with their 95% CIs were used to measure the strength of the relationship between tomato intake and prostate cancer risk. Some studies reported risk estimates for raw tomato and cooked tomato separately and did not report the effect of total tomato intake. In this situation, the study-specific RR in overall analysis was recalculated by pooling the risk estimates with the inverse-variance method37. A DerSimonian and Laird random-effects model38, which incorporates both within- and between-study variability, was applied to calculate the combined RR and its 95% CI. Subgroup analyses were carried out by geographical region, study design, study quality, and sample size.
Statistical heterogeneity among included studies was estimated using Cochran’s Q test and the I2 score39. The level of significancefor Cochran’s Q was test set at 0.1 (10%). The I2 score was adopted to evaluate the degree of heterogeneity (I2 < 25%: no heterogeneity; I2 = 25–50%: moderate heterogeneity; I2 > 50%: large or extreme heterogeneity).
A sensitivity analysis was conducted by omitting each study in turn and recalculating the pooled RR to test the impact of each study on the overall risk estimate. Potential publication bias was assessed through Begg’s test (rank correlation method)40 and Egger’s test (linear regression method)41. All statistical analyses were conducted with STATA 11.0 (StataCorp, College Station, TX), using two-sided P values (set at 0.05).
Additional Information
How to cite this article: Xu, X. et al. Tomato consumption and prostate cancer risk: a systematic review and meta-analysis. Sci. Rep. 6, 37091; doi: 10.1038/srep37091 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Story, E. N., Kopec, R. E., Schwartz, S. J. & Harris, G. K. An update on the health effects of tomato lycopene. Annu Rev Food Sci Technol 1, 189–210 (2010).
Li, X. & Xu, J. Dietary and circulating lycopene and stroke risk: a meta-analysis of prospective studies. Sci Rep 4, 5031 (2014).
Ried, K. & Fakler, P. Protective effect of lycopene on serum cholesterol and blood pressure: Meta-analyses of intervention trials. Maturitas 68, 299–310 (2011).
Wang, Y., Cui, R., Xiao, Y., Fang, J. & Xu, Q. Effect of Carotene and Lycopene on the Risk of Prostate Cancer: A Systematic Review and Dose-Response Meta-Analysis of Observational Studies. PLoS One 10, e0137427 (2015).
Chen, P. et al. Lycopene and Risk of Prostate Cancer: A Systematic Review and Meta-Analysis. Medicine (Baltimore) 94, e1260 (2015).
Rao, A. V. Processed tomato products as a source of dietary lycopene: bioavailability and antioxidant properties. Can J Diet Pract Res 65, 161–165 (2004).
Shahar, S. et al. Roles of diet, lifetime physical activity and oxidative DNA damage in the occurrence of prostate cancer among men in Klang Valley, Malaysia. Asian Pac J Cancer Prev 12, 605–611 (2011).
Salem, S. et al. Major dietary factors and prostate cancer risk: a prospective multicenter case-control study. Nutr Cancer 63, 21–27 (2011).
Subahir, M. N., Shah, S. A. & Zainuddin, Z. M. Risk factors for prostate cancer in Universiti Kebangsaan Malaysia Medical Centre: a case-control study. Asian Pac J Cancer Prev 10, 1015–1020 (2009).
Li, X. M. et al. Mass screening-based case-control study of diet and prostate cancer in Changchun, China. Asian J Androl 10, 551–560 (2008).
Jian, L., Du, C. J., Lee, A. H. & Binns, C. W. Do dietary lycopene and other carotenoids protect against prostate cancer? Int J Cancer 113, 1010–1014 (2005).
Bosetti, C. et al. Fraction of prostate cancer incidence attributed to diet in Athens, Greece. Eur J Cancer Prev 9, 119–123 (2000).
Jain, M. G., Hislop, G. T., Howe, G. R. & Ghadirian, P. Plant foods, antioxidants, and prostate cancer risk: findings from case-control studies in Canada. Nutr Cancer 34, 173–184 (1999).
Giovannucci, E. et al. Intake of carotenoids and retinol in relation to risk of prostate cancer. J Natl Cancer Inst 87, 1767–1776 (1995).
Mills, P. K., Beeson, W. L., Phillips, R. L. & Fraser, G. E. Cohort study of diet, lifestyle, and prostate cancer in Adventist men. Cancer 64, 598–604 (1989).
Diallo, A. et al. Associations between fruit, vegetable and legume intakes and prostate cancer risk: results from the prospective Supplementation en Vitamines et Mineraux Antioxydants (SU.VI.MAX) cohort. Br J Nutr 115, 1579–1585 (2016).
Hardin, J., Cheng, I. & Witte, J. S. Impact of consumption of vegetable, fruit, grain, and high glycemic index foods on aggressive prostate cancer risk. Nutr Cancer 63, 860–872 (2011).
Takachi, R. et al. Fruits and vegetables in relation to prostate cancer in Japanese men: the Japan Public Health Center-Based Prospective Study. Nutr Cancer 62, 30–39 (2010).
Ambrosini, G. L., de Klerk, N. H., Fritschi, L., Mackerras, D. & Musk, B. Fruit, vegetable, vitamin A intakes, and prostate cancer risk. Prostate Cancer Prostatic Dis 11, 61–66 (2008).
Stram, D. O. et al. Prostate cancer incidence and intake of fruits, vegetables and related micronutrients: the multiethnic cohort study* (United States). Cancer Causes Control 17, 1193–1207 (2006).
Darlington, G. A., Kreiger, N., Lightfoot, N., Purdham, J. & Sass-Kortsak, A. Prostate cancer risk and diet, recreational physical activity and cigarette smoking. Chronic Dis Can 27, 145–153 (2007).
Etminan, M., Takkouche, B. & Caamano-Isorna, F. The role of tomato products and lycopene in the prevention of prostate cancer: a meta-analysis of observational studies. Cancer Epidemiol Biomarkers Prev 13, 340–345 (2004).
WCRF-AICR continuous update project. The Associations between Food, Nutrition and Physical Activity and the Risk of Prostate Cancer, http://www.wcrf.org/sites/default/files/Prostate-Cancer-SLR-2014.pdf (2014).
Vlajinac, H., Ilic, M., Marinkovic, J. & Sipetic, S. Nutrition and prostate cancer. J BUON 15, 698–703 (2010).
Kirsh, V. A. et al. A prospective study of lycopene and tomato product intake and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 15, 92–98 (2006).
Sonoda, T. et al. A case-control study of diet and prostate cancer in Japan: possible protective effect of traditional Japanese diet. Cancer Sci 95, 238–242 (2004).
Hodge, A. M. et al. Foods, nutrients and prostate cancer. Cancer Causes Control 15, 11–20 (2004).
Norrish, A. E., Jackson, R. T., Sharpe, S. J. & Skeaff, C. M. Prostate cancer and dietary carotenoids. Am J Epidemiol 151, 119–123 (2000).
Kolonel, L. N. et al. Vegetables, fruits, legumes and prostate cancer: a multiethnic case-control study. Cancer Epidemiol Biomarkers Prev 9, 795–804 (2000).
Cohen, J. H., Kristal, A. R. & Stanford, J. L. Fruit and vegetable intakes and prostate cancer risk. J Natl Cancer Inst 92, 61–68 (2000).
Villeneuve, P. J., Johnson, K. C., Kreiger, N. & Mao, Y. Risk factors for prostate cancer: results from the Canadian National Enhanced Cancer Surveillance System. The Canadian Cancer Registries Epidemiology Research Group. Cancer Causes Control 10, 355–367 (1999).
Key, T. J., Silcocks, P. B., Davey, G. K., Appleby, P. N. & Bishop, D. T. A case-control study of diet and prostate cancer. Br J Cancer 76, 678–687 (1997).
Yang, C. M., Lu, I. H., Chen, H. Y. & Hu, M. L. Lycopene inhibits the proliferation of androgen-dependent human prostate tumor cells through activation of PPARgamma-LXRalpha-ABCA1 pathway. J Nutr Biochem 23, 8–17 (2012).
Elgass, S., Cooper, A. & Chopra, M. Lycopene treatment of prostate cancer cell lines inhibits adhesion and migration properties of the cells. Int J Med Sci 11, 948–954 (2014).
Wan, L. et al. Dietary tomato and lycopene impact androgen signaling- and carcinogenesis-related gene expression during early TRAMP prostate carcinogenesis. Cancer Prev Res (Phila) 7, 1228–1239 (2014).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151, 264–269, W264 (2009).
Woolf, B. On estimating the relation between blood group and disease. Ann Hum Genet 19, 251–253 (1955).
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials. Control Clin Trials 7, 177–188 (1986).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539–1558 (2002).
Begg, C. B. & Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 (1994).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997).
Acknowledgements
This study was supported by grants from the National Key Clinical Specialty Construction Project of China, Key Medical Disciplines of Zhejiang Province, Health Sector Scientific Research Special Project (201002010), Combination of Traditional Chinese and Western Medicine Key Disciplines of Zhejiang Province (2012-XK-A23), Zhejiang Province Key Project of Science and Technology (2014C04008-2), National Natural Science Foundation of China (81502215, 81472375, 81372773), Scientific Research Foundation of the Ministry of Public Health of China (WKJ2012-2-009).
Author information
Authors and Affiliations
Contributions
All authors contributed significantly to this work. X.X., X.Y.Z. and L.P.X. designed the research study; X.X., J.F.L., X.W. and Z.L. performed the research study and collected the data; X.X., S.W., S.M. and Y.Z. analyzed the data; X.X. and X.Y.Z. wrote the first draft of the manuscript; all authors reviewed, edited and approved the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Xu, X., Li, J., Wang, X. et al. Tomato consumption and prostate cancer risk: a systematic review and meta-analysis. Sci Rep 6, 37091 (2016). https://doi.org/10.1038/srep37091
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep37091
This article is cited by
-
Dietary tomato inhibits angiogenesis in TRAMP prostate cancer but is not protective with a Western-style diet in this pilot study
Scientific Reports (2021)
-
Dietary inflammatory index and the risk of prostate cancer: a dose-response meta-analysis
European Journal of Clinical Nutrition (2020)
-
Tomato consumption and intake of lycopene as predictors of the incidence of prostate cancer: the Adventist Health Study-2
Cancer Causes & Control (2020)
-
Differences in the prevalence of modifiable risk and protective factors for prostate cancer by race and ethnicity in the National Health and Nutrition Examination Survey
Cancer Causes & Control (2020)
-
Intra-articular dexmedetomidine in knee arthroscopy: A systematic review and meta-analysis
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.