Abstract
Drug-resistant Klebsiella pneumoniae, especially extended-spectrum β-lactamase (ESBL)- and/or AmpC β-lactamase-producing strains, is an emerging problem worldwide. However, few data focusing on drug susceptibility of K. pneumoniae from community is available. In this study, we analyzed 1016 K. pneumoniae isolates from outpatients or those visiting emergency rooms collected during 2002–2012 from Taiwan Surveillance of Antimicrobial Resistance program. Significantly decreased susceptibilities to 3rd generation cephalosporins and ciprofloxacin were found during the study period. By 2012, susceptibility to cefotaxime and ciprofloxacin was 83.6% and 81.6%, respectively. The prevalence of ESBL-producers increased from 4.8% in 2002 to 11.9% in 2012 (P = 0.012), while that of AmpC β-lactamase-producers increased from 0% to 9.5% in the same period (P < 0.001). Phylogenic analysis of the ESBL and AmpC-β-lactamase-producers by pulsed-field gel electrophoresis and multi-locus sequence typing revealed wide genetic diversity even among the most common sequence type 11 isolates (33.0%). By multivariate analysis, later study year, elderly, and urine isolates were associated with carriage of ESBL genes, while only urine isolates were associated with carriage of AmpC β-lactamase genes. Further studies are needed to determine which antibiotics are reasonable empirical therapy options for patients presenting with severe sepsis that might be caused by K. pneumoniae.
Similar content being viewed by others
Introduction
Klebsiella pneumoniae belongs to the family of Enterobacteriaceae. In addition to the ability to colonize gastrointestinal tract, nasopharynx, and skin, K. pneumoniae could cause various infection syndromes, including urinary tract infection, intra-abdominal infection, skin and soft tissue infection, and pneumonia, in both community and healthcare-associated settings1,2,3.
Treatment of bacterial infections depends heavily on effective antimicrobial therapy. Delayed use of effective antibiotics has been associated with a higher mortality rate in patients with severe infections4. Therefore, the presence of drug resistance in the infecting pathogen would adversely affect the treatment outcome5. One major drug resistance mechanism of concern in K. pneumoniae is the production of β-lactamases, especially extended-spectrum β-lactamases (ESBLs) and/or AmpC β-lactamases because these isolates are resistant to broad-spectrum cephalosporins and/or β-lactam/β-lactamase inhibitors6,7. In addition, these isolates are often resistant to several classes of non-β-lactam antibiotics.
The overall prevalence of ESBL-producing K. pneumoniae isolates varies widely in different studies, from 3.6% in Canada, 16% in U.S.A, to 26.2% in Korea, and 39.3% in Eastern Europe8,9,10,11,12. The Study for Monitoring Antimicrobial Resistance Trends (SMART) has shown that the prevalence of ESBL-producing K. pneumoniae isolates from intra-abdominal infection (IAI) was also high13. Of special concern also is that a trend of increased prevalence of ESBLs among K. pneumoniae has been observed globally, even in low prevalence countries such as Canada14. A similar trend has been noted in pediatric patients15,16. Data on the prevalence of AmpC β-lactamases carriage are less available, but an increased trend has also been observed from different studies17,18. Community-acquired ESBL K. pneumoniae infection has also emerged. One study from France showed that ESBL-producing strains accounted for 6.6% of community-onset K. pneumoniae urinary tract infections19. It has been recognized that community-based patients can be reservoirs for ESBL- and AmpC β-lactamase-producing strains, especially when they are from nursing home or clinics20.
In Taiwan, nosocomial ESBL K. pneumoniae infection has been a recognized emerging threat21,22. However, updated epidemiological and microbiological data about K. pneumoniae from community settings in Taiwan are still limited. Such data could impact empirical therapy regimen. The present study analyzed data on K. pneumoniae from community settings collected by the Taiwan Surveillance of Antimicrobial Resistance (TSAR) program from 2002 to 2012 with the goals of providing the aforementioned valuable information to update the suggestion of empirical antibiotics regimen.
Result
Between 2002 and 2012, 1016 non-duplicated K. pneumoniae isolates from TSAR III to VIII were included. The number of isolates from each study period was as follows: TSAR III (2002): 124, IV (2004): 149, V (2006): 152, VI (2008): 186, VII (2010): 195, VIII (2012): 210. A total of 37.2% (378) of the isolates were from blood samples, and 30.4% (309) were from urine. The remaining isolates were grouped as others (32.4%, 329). The mean age of the source patients was 60.6 ± 21.2 years, with age data missing in 20 people. The percentage of adults (19–64 y) and elderly (≥65 y) was 45.2% (450) and 49.2% (490), respectively. The proportion of pediatric patients (≤18y) was only 5.6% (56).
Antimicrobial susceptibilities of K. pneumoniae over study periods
For ease of comparison, we grouped TSAR III (2002) ~V (2006) as Period I (total isolates number = 425) and TSAR VI (2008) ~ VIII (2012) as Period II (total isolates number = 591). The susceptibilities of K. pneumoniae to different antimicrobial agents, including β-lactams and non-β-lactams, are listed in Table 1. Decreased susceptibilities from Period I to Period II were noted in most of the antimicrobial agents tested. The most significant decrease was observed in all 1st, 2nd, and 3rd -generation cephalosporins. However, 90.6% of isolates in Period I and 87.1% of isolates in Period II remained susceptible to amoxicillin/clavulanate. The susceptibilities to four different carbapenems were all well above 95%. Among non-β-lactams, significant decrease in susceptibility was observed in ciprofloxacin, and the susceptibilities to ciprofloxacin and levofloxacin in Period II were only 81.6% and 80.8%, respectively. Although the data was not available in period I, susceptibility to tigecycline was high (97.8%) in Period II.
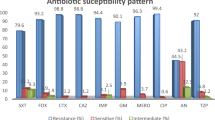
K. pneumoniae isolates from blood showed similar susceptibility pattern to those from specimens other than blood and urine (Table 2). However, isolates from urine were more resistant. For all cephalosporins and ciprofloxacin, the differences were significant. Among different age groups, isolates from elderly patients universally had higher rates of non-susceptibility compared to those from adult patients. For isolates from pediatric patients, rates of non-susceptibility were usually similar to or lower than those of adult patients, and all were lower than those of elderly patients (Fig. 1).
Susceptibilities of K. pneumoniae isolates to different antimicrobial agents among pediatric, adult, and elderly patients.
*The susceptibility is significantly lower among isolates from elderly compared with those from adult patients. (P < 0.05). #The susceptibility is significantly lower among isolates from elderly compared with those from pediatric patients. (P < 0.05).
Prevalence and susceptibility pattern of ESBL/AmpC β-lactamase-producers
A total of 138 isolates with aztreonam, ceftazidime, and/or cefotaxime MIC ≥ 2 mg/L were identified. All of them were subject to the CLSI ESBL phenotypic confirmatory test and detection of ESBL and AmpC β-lactamase genes by PCR. Among them, 27 isolates were positive for both ESBL and AmpC β-lactamase genes, 54 were positive for ESBL genes but negative for AmpC β-lactamase genes, 34 were positive for AmpC β-lactamase genes but negative for ESBL genes, and the remaining 23 isolates (901 isolates if those 878 with negative ESBL screening test are also pooled together) were negative for both ESBL and AmpC β-lactamase genes. No isolate with carbapenemase was detected. Rates of susceptibility were highest in isolates negative for ESBL and AmpC β-lactamase genes, followed by those with either ESBL or AmpC β-lactamase genes, and lowest in isolates carrying both ESBL and AmpC β-lactamase genes (Table 3). Of note, susceptibility to cefepime was significantly different between ESBL-positive group and ESBL-negative group, regardless of their AmpC β-lactamase gene status. Combining susceptible (S) and susceptible dose dependent (SDD) categories together, the susceptibility of cefepime was 99.9% in ESBL negative/AmpC β-lactamase negative group, but it was only 37% in ESBL-positive/AmpC β-lactamase-negative group. Co-resistance to aztreonam and ciprofloxacin could be observed among ESBL- and/or AmpC β-lactamase-producing strains. In addition, decreased susceptibility to carbapenems occurred among isolates with both ESBL and AmpC β-lactamase genes.
Between 2002 and 2012, ESBL-producers among our K. pneumoniae isolates increased from 4.8% to 11.9%, and AmpC β-lactamase-producers increased from 0% to 9.5%, both with p values of <0.05 in trend analysis (Table 4). The rate of cefotaxime non-susceptibility increased significantly from 5.7% to 18.7% during the study period (Fig. 2). Using cefotaxime non-susceptibility as predictors of ESBL- and/or AmpC β-lactamase-producers, the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) was 98.3%, 98.2%, 87.6%, and 99.8%, respectively. If ceftazidime was used instead, then the sensitivity, specificity, PPV and NPV was 84.5%, 98.7%, 89.0%, and 98.5%, respectively.
Among 81 ESBL-producers identified, 52 (64%) carried CTX-M-type genes, 24 (30%) carried SHV-type genes, and 5 (6%) carried both CTX-M-type and SHV-type genes. For AmpC β-lactamase-producers, 90% (55 out of 61) carried DHA-type genes, and 10% (6 out of 61) carried CMY-type genes. CTX-M plus DHA genes were the most common combination for isolates with both ESBL and AmpC β-lactamase genes, followed by the combination of SHV and DHA genes, at 59% and 41%, respectively.
Phylogenetic analysis of the ESBL and/or AmpC β-lactamase-producers
The phylogenetic analysis of the ESBL and/or AmpC β-lactamase-producers was determined by multi-locus sequence typing (MLST) and pulsed-filed gel electrophoresis (PFGE). Among the 115 isolates, the most prevalent sequence types (STs) was ST11 (38, 33.0%), followed by ST48 (10, 8.7%), ST15 (6, 5.2%), and ST378 (6, 5.2%). Another 45 isolates belonged to 36 additional STs, and the remaining 10 isolates belonged to new STs. PFGE results demonstrated great genetic diversity with only one small cluster of 15 isolates sharing ≥ 80% similarity in PFGE pattern (Fig. 3). Although these 15 isolates all belonged to ST11, they were recovered from 11 hospitals (data not shown) in different study years and some carried different ESBL and/or AmpC β-lactamase genes combinations, and only 2 isolates from 2008 had indistinguishable PFGE patterns but one isolate carried CTX-M-type ESBL, while the other carried CTX-M-type ESBL and DHA-type AmpC-β-lactamase genes.
Dendrogram of XbaI-digested genomic DNA of Klebsiella pneumoniae isolates with ESBL and/or AmpC β-lactamase genes.
*PFGE of 11 of the 115 isolates failed PFGE. The ST of these 11 isolates are ST11 (3), ST48 (2), and 1 each of ST23, ST48, ST65, ST378, ST750, ST897; Source: U, urine; B, blood; O, others. New ST, isolates with new allele profiles not in the MLST database.
Factors associated with carriage of ESBL and/or AmpC β-lactamase genes
In univariate analysis, study years, age groups, and specimen types were associated with carriage of ESBL and/or AmpC β-lactamase genes. By multivariate analysis, study year (2004 vs. 2012), age group (elderly vs. pediatric), and specimen types (urine vs. blood or others) remained factors associated with carriage of ESBL genes, while only specimen types (urine vs. blood or others) was associated with carriage of AmpC β-lactamase genes (Table 5).
Discussion
The present study analyzed data on K. pneumoniae from a biennial nationwide surveillance program between 2002 and 2012 in Taiwan focusing on community-sourced isolates. Our data revealed decreased susceptibilities to most β-lactam antibiotics and fluoroquinolones during 2008–2012 (Period II) compared with those during 2002–2006 (Period I). For period II, susceptibilities to 1st, 2nd, and 3rd generation cephalosporins were all lower than 90%, and the susceptibility to ciprofloxacin was 81.6%. Urine isolates in the present study tended to be less susceptible to most antibiotics than blood isolates. Furthermore, compared with studies focusing on community-acquired K. pneumoniae urinary tract infections from other countries, lower susceptibility of urine isolates was also observed in our study. For example, the susceptibility of our urine isolates was 79.3% to cefotaxime (92.8% in USA), 85.7% to cefepime (94% in U.S.A., and 97.7% in Japan), 84.4% for piperacillin-tazobactam (91% in U.S.A., and 97.7% in Japan), and 73.0% for ciprofloxacin (91.6% in U.S.A., 77.3% in Korea, and 91.7% in Japan.)23,24,25.
In our study, the overall percentage of ESBL-producing K. pneumoniae isolates was 7.6% but the prevalence of ESBL-producers increased significantly over the study years, from 4.8% in 2002 to 11.9% in 2012. In other countries, the prevalence of ESBL-producers was 7.2% among K. pneumoniae urine isolates causing community-acquired urinary tract infection in the United States23, 3.3% for community-onset K. pneumoniae bacteremia in Korea26, and only 3.6% in Canada even when both community-acquired and hospital-acquired infection isolates were included8. Compared with the studies aforementioned, our study revealed that the prevalence of ESBL-producers among community-acquired K. pneumoniae infections was higher in Taiwan. Furthermore, the present study also showed an increasing prevalence of AmpC β-lactamase-producers (from 0% in 2002 to 9.5% in 2012). K. pneumoniae carrying ESBL and/or AmpC β-lactamase genes usually also had higher rates of resistance to non-β-lactam antibiotics. For example, susceptibility to amikacin was 64.8% in isolates carrying ESBL genes and 67.7% in isolates carrying AmpC β-lactamase genes, compared to 99.6% in isolates with neither ESBL nor AmpC β-lactamase genes. Similarly, susceptibility to ciprofloxacin was 33.3% and 38.2% in isolates with ESBL and AmpC β-lactamase genes, respectively, while it was 92.5% in isolates with neither. For isolates with both ESBL and AmpC β-lactamase genes, susceptibility to amikacin and ciprofloxacin was only 25.9% and 0%, respectively, at rates similar to those previously reported23,27.
The high ciprofloxacin resistance rate among ESBL-positive or AmpC β-lactamase-positive isolates is alarming. Of special concern is that an earlier study conducted between 1998 and 2000 in Taiwan demonstrated that the ciprofloxacin resistance rate among ESBL-producing K. pneumoniae was 18.5%, which is much lower than that shown in our present study (77.8%)28. Because fluoroquinolones are important alternative antibiotics to carbapenems, and they are the antibiotics that have oral forms for treating ESBL-producers, the increased fluoroquinolone resistance raises great clinical concern. Kang and colleagues reported that prior use of fluoroquinolones could be an important independent risk factor for ciprofloxacin resistance in ESBL-producing K. pneumoniae29. Fluoroquinolone consumption in Taiwan has increased over the years30. From our study, overall susceptibility to ciprofloxacin was still high among pediatric patients (98.2%) but was only 78.6% among elderly patients, which would be an indirect evidence of heavy and cumulated fluoroquinolone exposure with age among the general population in Taiwan. Control of fluoroquinolone consumption is mandatory to counteract the continuous increase of fluoroquinolone resistance.
Another worrisome finding was the diminished susceptibilities of carbapenems among isolates carrying both ESBL and AmpC β-lactamase genes. In our study, while the overall susceptibility to carbapenems for K. pneumoniae was high, the rates of susceptibility to ertapenem and imipenem was only 57.1% and 63%, respectively, in isolates positive for both ESBL and AmpC β-lactamase genes. Altered outer membranous permeability, such as porin mutation (OmpK35 or OmpK36 loss), coupled with the presence of ESBLs or AmpC β-lactamases, and the production of carbapenemases are two major mechanisms for carbapenem resistance in K. pneumoniae in Taiwan27,31. Since no carbapenemase-producers were detected in the present study, mutations in OmpK 35/36 plus carriage of ESBLs or AmpC β–lactamases are likely the major mechanism that led to the carbapenem resistance in our isolates.
In the present study, the predominant ESBL genes were CTX-M-type (64%), and most of AmpC β-lactamase genes belonged to DHA-type (90%). Previous studies had shown that CTX-M-type genes are currently the most common type of ESBL genes worldwide, and it is the same for community-sourced ESBL K. pneumoniae strains7,20. CMY-type gene was the most common AmpC β-lactamase gene among Enterobacteriaceae isolates in Asia-Pacific region, according to results from the SMART study32. However, several studies from Korea also demonstrated that DHA was the predominant type of AmpC β-lactamase gene among their K. pneumoniae isolates12,18. Together with our results, these results indicated a geographical distribution difference of endemic AmpC β-lactamase genes.
Phylogenetic analysis by MLST showed that ST11, ST48, ST15, and ST378 were the most common sequence types among our ESBL- and/or AmpC β-lactamase-producing K. pneumoniae isolates. However, these four major sequence types comprised only 52.2% of isolates, with 33.0% being ST11. A separate study focusing on imipenem-non-susceptible K. pneumoniae isolates during similar period in Taiwan also found ST11 and ST48 to be the dominant strains33. Globally, the epidemiological data on K. pneumoniae genotype is limited, but ST11 has been found to be one of the pandemic clones in Europe and Asia34,35. ST11 strains have also been associated with multi-drug resistance in a previous study36. Therefore, isolates of ST11 may be more prone to acquire resistance. The underlying mechanisms need further investigation. By PFGE, the ESBL and/or AmpC β-lactamase-producers showed a great diversity, and only one small cluster (15 isolates only) of ST11 isolates was found. However, in addition to having 3 three different combinations of ESBL and AmpC β-lactamase genes, only 2 of the 15 isolates in the cluster had indistinguishable PFGE patterns. Taken together, our molecular study results indicated that the increased prevalence of ESBL and/or AmpC-β-lactamase-producing K. pneumoniae was likely mostly due to transmission of the β-lactamase genes via horizontal gene transfer elements such as plasmids instead of clonal spread.
Using the 2014 CLSI breakpoints, 98.3% of our ESBL- and/or AmpC β-lactamase- positive isolates were non-susceptible to cefotaxime, while 84.5% of them were non-susceptible to ceftazidime. These findings are similar to our previous study on ESBL E. coli37. Cefotaxime was more sensitive than ceftazidime in identifying ESBL- and/or AmpC β-lactamase-producers in our K. pneumoniae isolates (98.3% vs. 84.5%). These results would be partly attributed to the predominance of CTX-M-type genes, the enzymes of which hydrolyze cefotaxime more efficiently than ceftazidime38.
The findings from our study have important clinical implications. It has been suggested that the empirical antibiotics therapy for severely infected patients should cover at least 90% of all possible bacterial pathogens39. Based on the present study, however, susceptibility to 3rd generation cephalosporins and fluoroquinolones in our K. pneumoniae isolates was both below 90%, and these rates were even lower among isolates from elderly patients, at 81.2% for cefotaxime and 78.6% for ciprofloxacin. Besides, the prevalence of ESBLs-producers has reached 11.9% in 2012, indicating that 3rd generation cephalosporins would no longer be reliable options for empirical therapy. Those antibiotics with higher susceptibility rates, especially carbapenems, to which over 95% of the isolates from all age groups were susceptible, would be more reasonable options for empirical therapy in critical conditions if K. pneumoniae infection, even from community settings, is suspected.
In the multivariate analysis, age, isolates from later study years, and isolates from urine were independent factors associated with ESBL-producing K. pneumoniae. Elderly patients might have more exposure to medical care, long-term care facilities, and antibiotics. Therefore, they would be at a higher risk of acquiring drug-resistant bacteria. As for AmpC β-lactamase-producers, recovery from urine was an independent factor. The reason that isolates from later study period was not an independent factor associated with AmpC β-lactamase-producers may be due to the limitation of statistical method itself. This is because no AmpC β-lactamase-producers were detected in TSAR III (2002), which led to a divergent estimation of regression coefficient for TSAR III (2002) in the multivariate regression model and in turn made the statistical result non-significant. However, by chi-square for trend analysis, isolates from later study period had significantly higher probability of being AmpC β-lactamase-producers. Therefore, we believe that isolates from later study period was a significant risk factor associated with AmpC β-lactamase-producers. The reasons for the strong association of urine isolates with ESBL- and/or AmpC β-lactamase-producers need further investigation.
Our study has several limitations. First, we have limited clinical information on the source patients. Therefore, we could not identify whether there were other independent factors, such as prior hospitalization, recent antibiotics use, and healthcare facility exposure, for ESBL- and/or AmpC β-lactamase-producers. However, age was an independent factor for ESBL-producers, thus prior hospitalization, and antibiotics as well as healthcare facility exposure were likely associated factors. Second, the isolates were collected biennially during a three-month period only. However, the enrolled isolates were from 25–28 hospitals over four geographical areas of Taiwan. Therefore, we consider that the results described here are representative for K. pneumoniae from community settings in Taiwan. Finally, the actual location of the ESBL and AmpC β-lactamase genes were not determined in our study. Although it has been shown that ESBL genes could reside on the chromosome40, several studies on ESBL-producing K. pneumoniae have found CTX-M-type and SHV-type ESBL genes to be mostly located on plasmids41,42,43,44. Furthermore, the great genotypic diversity of our ESBL and/or AmpC β-lactamase-producers, having different combinations of ESBL/AmpC β-lactamase genes even among isolates within the same PFGE cluster, implied that these resistant genes are acquired through transfer of plasmids among isolates of different genetic backgrounds.
In conclusion, our multicenter surveillance for K. pneumoniae isolates from community settings in Taiwan between 2002 and 2012 revealed decreased susceptibilities to most antibiotics, especially 3rd generation cephalosporins and fluoroquinolones. The prevalence of ESBL- and AmpC β-lactamase-producers had also increased, indicating that the chance of encountering multidrug-resistant pathogens would increase even in the scenario of community-sourced infection. Co-resistance to other antibiotics, especially ciprofloxacin, among ESBL- and/or AmpC β-lactamase-producing K. pneumoniae is of particular concern since treatment options would be further limited. These results indicate that 3rd generation cephalosporins may no longer be reliable empirical treatment choices, and further studies are needed to determine which antibiotics are current reasonable options when a patient presents with severe sepsis which might be caused by K. pneumoniae.
Method
As part of the TSAR program, K. pneumoniae isolates were collected biennially from 2002 (TSAR III) to 2012 (TSAR VIII). During the collection year, isolates were collected from July to September from 25–28 regional hospitals and medical centers located in different geographical regions of Taiwan. Detailed collection protocol has been published previously45. All isolates were stored at −70 °C. In present study, we only enrolled K. pneumoniae clinical isolates from patients visiting emergency rooms or from outpatient clinics. Written informed consent was not obtained since all the isolates were recovered from clinical samples taken as part of standard care and the patient information was anonymized prior to analysis. TSAR program was approved by the Research Ethics Committee of National Health Research Institutes (NHRI), Taiwan (EC960205 and EC1010602-E), and was conducted in accordance with the principles of Declaration of Helsinki and the International Conference on Harmonization for Good Clinical Practice.
Isolate identification
Isolates reported as K. pneumoniae were subcultured to blood agar and MacConkey agar plates for purity check. Species identification was confirmed at NHRI based on colony morphology, conventional biochemical reactions, and use of Vitek II GN cards (bioMérieux, Marcy l′Etoile, France).
Antimicrobial susceptibility testing (AST)
Minimum inhibitory concentrations (MICs) were determined using reference broth microdilution method following the guidelines of the manufacturer and Clinical and Laboratory Standards Institute (CLSI) 201446. Sensititre custom-designed plates were used from TSAR III (2002) to TSAR VI (2008), and the standard GNX2F plates were used in TSAR VII (2010) and TSAR VIII (2012) [ThermoFisher Scientific (formerly Trek Diagnostics), East Grinstead, UK]. All isolates were subcultured twice on sheep blood agar plates from −70 °C prior to AST. Quality control was performed each day using Escherichia coli ATCC 25922, Escherichia coli ATCC 35218, Klebsiella pneumoniae ATCC 700603, and Pseudomonas aeruginosa ATCC 27853.
The following agents were tested on isolates from all study years: amikacin, ampicillin, aztreonam, cefazolin, cefepime, cefotaxime, cefoxitin, ceftazidime, cefuroxime, ciprofloxacin, gentamicin, and imipenem. Other agents not tested in all years included (years tested) amoxicillin/clavulanate (2002–2008), ertapenem (2012), piperacillin (2002–2010), tetracycline (2002–2008), and tigecycline (2010 and 2012). Interpretive criteria are based on the 2014 CLSI breakpoints46. Susceptibility to tigecycline was interpreted using breakpoints proposed by the European Committee on Antimicrobial Susceptibilities Testing (EUCAST) (http://www.eucast.org/clinical_breakpoints/)
Detections of ESBL, AmpC β-lactamase, and carbapenemase genes
The ESBL screening test-positive isolates were defined as isolates with aztreonam, ceftazidime, or cefotaxime MIC ≥ 2 mg/L according to the suggestions of CLSI46. The CLSI ESBL confirmatory test was performed on all isolates positive for ESBL screening test using cefotaxime and ceftazidime disks with and without clavulanate46. These ESBL screening test-positive isolates were also subject to detection of ESBL and/or AmpC β-lactamase genes by multiplex PCR using primers and following protocols that were previously described47,48. The DNA product of the blaSHV gene was subject to NheІ restriction enzyme digestion to differentiate non-ESBL SHV and SHV-ESBL49. Isolates non-susceptible to carbapenem were tested by carbapenemase PCR using published methods50.
Multi-locus sequence typing (MLST) and pulsed-field gel elelctrophoresis (PFGE)
Molecular typing of ESBL- and/or AmpC β-lactamase-producers was performed using MLST and PFGE following previously published protocols and information from the MLST website (http://bigsdb.pasteur.fr)51. For interpretation of the PFGE banding patterns, unweighted-pair group method using average linkages (UPGMA) dendrograms were constructed from the original data. Isolates that exhibited similarity of 80% or greater of their banding patterns were considered to belong to the same cluster if more than 3 isolates were present52.
Data analysis
Susceptibility interpretation analysis was made using the WHONET software53. Duplicate isolates were excluded before analysis. TSAR III-V (2002–2006) and TSAR VI–VIII (2008–2012) were grouped as Period I and II, respectively, for the sake of comparison. Intermediate susceptibility and resistance were grouped together as ”non-susceptibility”. Categorical variables were compared using chi-square test or Fisher’s exact test (if the number was less than 10). If statistical difference was obtained after comparing categorical variables with three different levels, post-hoc analysis was then performed to identify which level was significantly different from the others. Trend analysis was made using chi-square. Multivariable logistic regression analysis was performed to assess the variables (including study year, specimen type, and patient age group) among ESBL- and/or AmpC β-lactamase-producers vs. non-producers. SAS 9.2 (SAS Institute, Cary, NC, USA) was used for the above analyses. A 2-tailed P value less than 0.05 was considered statistically significant.
Additional Information
How to cite this article: Lin, W.-P. et al. The Antimicrobial Susceptibility of Klebsiella pneumoniae from Community Settings in Taiwan, a Trend Analysis. Sci. Rep. 6, 36280; doi: 10.1038/srep36280 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Pitout, J. D., Nordmann, P. & Poirel, L. Carbapenemase-Producing Klebsiella pneumoniae, a Key Pathogen Set for Global Nosocomial Dominance. Antimicrobial agents and chemotherapy 59, 5873–5884, 10.1128/aac.01019-15 (2015).
Melot, B., Colot, J. & Guerrier, G. Bacteremic community-acquired infections due to Klebsiella pneumoniae: clinical and microbiological presentation in New Caledonia, 2008–2013. International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases 41, 29–31, 10.1016/j.ijid.2015.10.013 (2015).
Broberg, C. A., Palacios, M. & Miller, V. L. Klebsiella: a long way to go towards understanding this enigmatic jet-setter. F1000prime reports 6, 64, 10.12703/p6-64 (2014).
Harbarth, S. et al. Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. The American journal of medicine 115, 529–535 (2003).
Brolund, A. Overview of ESBL-producing Enterobacteriaceae from a Nordic perspective. Infection ecology & epidemiology 4, 10.3402/iee.v4.24555 (2014).
Lee, J. A. et al. Epidemiollogy and clinical features of community-onset bacteremia caused by extended-spectrum beta-lactamase-producing Klebsiella pneumoniae. Microbial drug resistance (Larchmont, N.Y.) 17, 267–273, 10.1089/mdr.2010.0134 (2011).
Pitout, J. D. Enterobacteriaceae that produce extended-spectrum beta-lactamases and AmpC beta-lactamases in the community: the tip of the iceberg? Current pharmaceutical design 19, 257–263 (2013).
Karlowsky, J. A. et al. In vitro activity of ceftaroline-avibactam against gram-negative and gram-positive pathogens isolated from patients in Canadian hospitals from 2010 to 2012: results from the CANWARD surveillance study. Antimicrobial agents and chemotherapy 57, 5600–5611, 10.1128/aac.01485-13 (2013).
Castanheira, M., Farrell, S. E., Krause, K. M., Jones, R. N. & Sader, H. S. Contemporary diversity of beta-lactamases among Enterobacteriaceae in the nine U.S. census regions and ceftazidime-avibactam activity tested against isolates producing the most prevalent beta-lactamase groups. Antimicrobial agents and chemotherapy 58, 833–838, 10.1128/aac.01896-13 (2014).
Balode, A., Punda-Polic, V. & Dowzicky, M. J. Antimicrobial susceptibility of gram-negative and gram-positive bacteria collected from countries in Eastern Europe: results from the Tigecycline Evaluation and Surveillance Trial (T.E.S.T.) 2004–2010. International journal of antimicrobial agents 41, 527–535, 10.1016/j.ijantimicag.2013.02.022 (2013).
Carbonne, A. et al. National multidrug-resistant bacteria (MDRB) surveillance in France through the RAISIN network: a 9 year experience. The Journal of antimicrobial chemotherapy 68, 954–959, 10.1093/jac/dks464 (2013).
Li, X. M. et al. [Frequency of extended-spectrum beta-lactamase (ESBL) and AmpC beta-lactamase genes in Escherichia coli and Klebsiella pneumoniae over a three-year period in a University Hospital in Korea]. The Korean journal of laboratory medicine 30, 616–623, 10.3343/kjlm.2010.30.6.616 (2010).
Kazmierczak, K. M. et al. Characterization of extended-spectrum beta-lactamases and antimicrobial resistance of Klebsiella pneumoniae in intra-abdominal infection isolates in Latin America, 2008–2012. Results of the Study for Monitoring Antimicrobial Resistance Trends. Diagnostic microbiology and infectious disease 82, 209–214, 10.1016/j.diagmicrobio.2015.03.025 (2015).
Denisuik, A. J. et al. Molecular epidemiology of extended-spectrum beta-lactamase-, AmpC beta-lactamase- and carbapenemase-producing Escherichia coli and Klebsiella pneumoniae isolated from Canadian hospitals over a 5 year period: CANWARD 2007–11. The Journal of antimicrobial chemotherapy 68 Suppl 1, i57–i65, 10.1093/jac/dkt027 (2013).
Chandramohan, L. & Revell, P. A. Prevalence and molecular characterization of extended-spectrum-beta-lactamase-producing Enterobacteriaceae in a pediatric patient population. Antimicrobial agents and chemotherapy 56, 4765–4770, 10.1128/aac.00666-12 (2012).
Murray, T. S. & Peaper, D. R. The contribution of extended-spectrum beta-lactamases to multidrug-resistant infections in children. Current opinion in pediatrics 27, 124–131, 10.1097/mop.0000000000000182 (2015).
Miro, E. et al. Prevalence and molecular epidemiology of acquired AmpC beta-lactamases and carbapenemases in Enterobacteriaceae isolates from 35 hospitals in Spain. European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology 32, 253–259, 10.1007/s10096-012-1737-0 (2013).
Park, M. J. et al. An Increase in the clinical isolation of acquired AmpC beta-lactamase-producing Klebsiella pneumoniae in Korea from 2007 to 2010. Annals of laboratory medicine 33, 353–355, 10.3343/alm.2013.33.5.353 (2013).
Martin, D. et al. Prevalence of extended-spectrum beta-lactamase producing Escherichia coli in community-onset urinary tract infections in France in 2013. The Journal of infection, 10.1016/j.jinf.2015.11.009 (2015).
Hanson, N. D. et al. Surveillance of community-based reservoirs reveals the presence of CTX-M, imported AmpC, and OXA-30 beta-lactamases in urine isolates of Klebsiella pneumoniae and Escherichia coli in a U.S. community. Antimicrobial agents and chemotherapy 52, 3814–3816, 10.1128/aac.00877-08 (2008).
Yang, C. C. et al. Nosocomial extended-spectrum beta-lactamase-producing Klebsiella pneumoniae bacteremia in hemodialysis patients and the implications for antibiotic therapy. International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases 28, 3–7, 10.1016/j.ijid.2014.07.012 (2014).
Liu, H. C. et al. Antimicrobial susceptibility of clinical Enterobacteriaceae isolates at the emergency department in a regional hospital: A threat of extended spectrum beta-lactamase-producers among nursing home residents. Journal of microbiology, immunology, and infection = Wei mian yu gan ran za zhi, 10.1016/j.jmii.2015.10.001 (2015).
Bouchillon, S. K., Badal, R. E., Hoban, D. J. & Hawser, S. P. Antimicrobial susceptibility of inpatient urinary tract isolates of gram-negative bacilli in the United States: results from the study for monitoring antimicrobial resistance trends (SMART) program: 2009-2011. Clinical therapeutics 35, 872–877, 10.1016/j.clinthera.2013.03.022 (2013).
Lee, D. S. et al. Antimicrobial susceptibility pattern and epidemiology of female urinary tract infections in South Korea, 2010–2011. Antimicrobial agents and chemotherapy 57, 5384–5393, 10.1128/aac.00065-13 (2013).
Ishikawa, K. et al. Japanese nationwide surveillance in 2011 of antibacterial susceptibility patterns of clinical isolates from complicated urinary tract infection cases. Journal of infection and chemotherapy: official journal of the Japan Society of Chemotherapy 21, 623–633, 10.1016/j.jiac.2015.05.014 (2015).
Lee, S., Han, S. W., Kim, K. W., Song do, Y. & Kwon, K. T. Third-generation cephalosporin resistance of community-onset Escherichia coli and Klebsiella pneumoniae bacteremia in a secondary hospital. The Korean journal of internal medicine 29, 49–56, 10.3904/kjim.2014.29.1.49 (2014).
Hawser, S. P. et al. Susceptibility of gram-negative aerobic bacilli from intra-abdominal pathogens to antimicrobial agents collected in the United States during 2011. The Journal of infection 68, 71–76, 10.1016/j.jinf.2013.09.001 (2014).
Yu, W. L. et al. Molecular epidemiology of extended-spectrum beta-lactamase-producing, fluoroquinolone-resistant isolates of Klebsiella pneumoniae in Taiwan. Journal of clinical microbiology 40, 4666–4669 (2002).
Kang, C. I. et al. Risk Factors for Ciprofloxacin Resistance in Bloodstream Infections Due to Extended-Spectrum beta-Lactamase-Producing Escherichia coli and Klebsiella pneumoniae. Microbial drug resistance (Larchmont, N.Y.) 10, 71–76, 10.1089/107662904323047835 (2004).
Su, C. H. et al. Increase of carbapenem-resistant Acinetobacter baumannii infection in acute care hospitals in Taiwan: association with hospital antimicrobial usage. PloS one 7, e37788, 10.1371/journal.pone.0037788 (2012).
Chiu, S. K. et al. National surveillance study on carbapenem non-susceptible Klebsiella pneumoniae in Taiwan: the emergence and rapid dissemination of KPC-2 carbapenemase. PloS one 8, e69428, 10.1371/journal.pone.0069428 (2013).
Sheng, W. H., Badal, R. E. & Hsueh, P. R. Distribution of extended-spectrum beta-lactamases, AmpC beta-lactamases, and carbapenemases among Enterobacteriaceae isolates causing intra-abdominal infections in the Asia-Pacific region: results of the study for Monitoring Antimicrobial Resistance Trends (SMART). Antimicrobial agents and chemotherapy 57, 2981–2988, 10.1128/aac.00971-12 (2013).
Ma, L., Lu, P. L., Siu, L. K. & Hsieh, M. H. Molecular typing and resistance mechanisms of imipenem-non-susceptible Klebsiella pneumoniae in Taiwan: results from the Taiwan surveillance of antibiotic resistance (TSAR) study, 2002–2009. Journal of medical microbiology 62, 101–107, 10.1099/jmm.0.050492-0 (2013).
Melegh, S. et al. Identification and characterization of CTX-M-15 producing Klebsiella pneumoniae clone ST101 in a Hungarian university teaching hospital. Acta microbiologica et immunologica Hungarica 62, 233–245, 10.1556/030.62.2015.3.2 (2015).
Hrabak, J. et al. International clones of Klebsiella pneumoniae and Escherichia coli with extended-spectrum beta-lactamases in a Czech hospital. Journal of clinical microbiology 47, 3353–3357, 10.1128/jcm.00901-09 (2009).
Andrade, L. N. et al. Expansion and evolution of a virulent, extensively drug-resistant (polymyxin B-resistant), QnrS1-, CTX-M-2-, and KPC-2-producing Klebsiella pneumoniae ST11 international high-risk clone. Journal of clinical microbiology 52, 2530–2535, 10.1128/jcm.00088-14 (2014).
Wang, J. T. et al. Antimicrobial Non-Susceptibility of Escherichia coli from Outpatients and Patients Visiting Emergency Rooms in Taiwan. PloS one 10, e0144103, 10.1371/journal.pone.0144103 (2015).
Rossolini, G. M., D’Andrea, M. M. & Mugnaioli, C. The spread of CTX-M-type extended-spectrum beta-lactamases. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases 14 Suppl 1, 33–41, 10.1111/j.1469-0691.2007.01867.x (2008).
Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. American journal of respiratory and critical care medicine 171, 388–416, 10.1164/rccm.200405-644ST (2005).
Huang, S. Y. et al. Analysis of the drug-resistant characteristics of Klebsiella pneumoniae isolated from the respiratory tract and CTX-M ESBL genes. Genetics and molecular research: GMR 14, 12043–12048, 10.4238/2015.October.5.17 (2015).
Bojer, M. S. et al. Concurrent emergence of multidrug resistance and heat resistance by CTX-M-15-encoding conjugative plasmids in Klebsiella pneumoniae. APMIS: acta pathologica, microbiologica, et immunologica Scandinavica 120, 699–705, 10.1111/j.1600-0463.2012.02885.x (2012).
Damjanova, I. et al. Dissemination of ST274 Klebsiella pneumoniae epidemic clone in newborn and adult hospital settings harbouring SHV-2A or CTX-M-15 type extended spectrum beta-lactamases-producing known plasmids. European journal of microbiology & immunology 1, 223–227, 10.1556/EuJMI.1.2011.3.6 (2011).
Chagas, T. P. et al. Diversity of genotypes in CTX-M-producing Klebsiella pneumoniae isolated in different hospitals in Brazil. The Brazilian journal of infectious diseases: an official publication of the Brazilian Society of Infectious Diseases 15, 420–425 (2011).
Liakopoulos, A., Mevius, D. & Ceccarelli, D. A Review of SHV Extended-Spectrum beta-Lactamases: Neglected Yet Ubiquitous. Frontiers in microbiology 7, 1374, 10.3389/fmicb.2016.01374 (2016).
Wang, J. T. et al. Antimicrobial susceptibilities of Proteus mirabilis: a longitudinal nationwide study from the Taiwan surveillance of antimicrobial resistance (TSAR) program. BMC infectious diseases 14, 486, 10.1186/1471-2334-14-486 (2014).
CLSI Performance Standards for Antimicrobial Susceptibility Testing: 24th Informational Supplement M100–S24. CLSI, Wayne, PA, USA (2014).
Monstein, H. J. et al. Multiplex PCR amplification assay for the detection of blaSHV, blaTEM and blaCTX-M genes in Enterobacteriaceae. APMIS: acta pathologica, microbiologica, et immunologica Scandinavica 115, 1400–1408, 10.1111/j.1600-0463.2007.00722.x (2007).
Perez-Perez, F. J. & Hanson, N. D. Detection of plasmid-mediated AmpC beta-lactamase genes in clinical isolates by using multiplex PCR. Journal of clinical microbiology 40, 2153–2162 (2002).
Nuesch-Inderbinen, M. T., Hachler, H. & Kayser, F. H. Detection of genes coding for extended-spectrum SHV beta-lactamases in clinical isolates by a molecular genetic method, and comparison with the E test. European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology 15, 398–402 (1996).
Queenan, A. M. & Bush, K. Carbapenemases: the versatile beta-lactamases. Clinical microbiology reviews 20, 440–458, table of contents, 10.1128/cmr.00001-07 (2007).
Diancourt, L., Passet, V., Verhoef, J., Grimont, P. A. & Brisse, S. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. Journal of clinical microbiology 43, 4178–4182, 10.1128/jcm.43.8.4178-4182.2005 (2005).
Wang, J. T. et al. Carbapenem-nonsusceptible Enterobacteriaceae in Taiwan. PloS one 10, e0121668, 10.1371/journal.pone.0121668 (2015).
O’Brien, T. F. & Stelling, J. Integrated Multilevel Surveillance of the World’s Infecting Microbes and Their Resistance to Antimicrobial Agents. Clinical microbiology reviews 24, 281–295, 10.1128/cmr.00021-10 (2011).
Acknowledgements
We thank the following 28 hospitals for participating in the Taiwan Surveillance of Antimicrobial Resistance (TSAR) program between 2002 and 2012: Buddhist Tzu Chi General Hospital, Cathay General Hospital, Changhua Christian Hospital, Cheng-Ching Hospital, Chung Shan Medical University Hospital, Ditmanson Medical Foundation Chia-Yi Christian Hospital, Da Chien Health Medical System (TSAR VIII only), Far Eastern Memorial Hospital, Hua-Lien Hospital, Jen-Ai Hospital, Kaohsiung Armed Forces General Hospital, Kaohsiung Chang Gung Memorial Hospital of the C. G. M. F, Kaohsiung Medical University Chung-Ho Memorial Hospital, Kaohsiung Veterans General Hospital, Kuang Tien General Hospital, Lo-Hsu Foundation Inc., Lotung Poh-Ai Hospital, Mennonite Christian Hospital, Min-Sheng Healthcare, National Cheng Kung University Hospital, Saint Mary’s Hospital Luodong, Show Chwan Memorial Hospital, Tungs’ Taichung MetroHarbor Hospital, Taichung Veterans General Hospital, Tainan Sin-Lau Hospital - the Presbyterian Church in Taiwan, Taipei City Hospital Heping Fuyou Branch, Taipei City Hospital Zhongxiao Branch (not TSAR VIII), Taipei Veterans General Hospital (TSAR VIII only), Tri-Service General Hospital (not TSAR V). This project was supported by an intramural grant from the National Health Research Institutes (IV-101-PP-01 and IV-102-PP-01).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: J.-T.W., S.-C.C. and T.-L.L. Performed the experiments: Y.-R.S., M.-C.T., H.-Y.W., J.-F.L. and I.-W.H. Contributed reagents/materials/analysis tools: J.-T.W., F.-Y.C., C.-P.F. and Y.-S.C. Analyzed the data: J.-T.W., S.-C.C., F.-Y.C., C.-P.F., Y.-C.C., Y.-S.C., Y.-R.S. and T.-L.L. Wrote the paper: W.-P.L., J.-T.W. and T.-L.L.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Lin, WP., Wang, JT., Chang, SC. et al. The Antimicrobial Susceptibility of Klebsiella pneumoniae from Community Settings in Taiwan, a Trend Analysis. Sci Rep 6, 36280 (2016). https://doi.org/10.1038/srep36280
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep36280
This article is cited by
-
Comparative genomic analysis of plasmids encoding metallo-β-lactamase NDM-5 in Enterobacterales Korean isolates from companion dogs
Scientific Reports (2022)
-
Multidrug-resistant Klebsiella pneumoniae: a retrospective study in Manaus, Brazil
Archives of Microbiology (2022)
-
Carbapenem Nonsusceptible Klebsiella pneumoniae in Taiwan: Dissemination and Increasing Resistance of Carbapenemase Producers During 2012–2015
Scientific Reports (2018)
-
A Novel Target Pathogen Identification and Tracking System Using Capillary Electrophoresis-Random Amplified Polymorphic DNA
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.