Abstract
Controversial results of the association between maternal body mass index (BMI) and risk of autism spectrum disorder (ASD) in offspring were reported among several studies. This meta-analysis was conducted to estimate the overall association between maternal BMI and risk of ASD in offspring. PubMed, EMBASE, Web of Science, and the Cochrane Library were searched until January 2016. Cohort and case-control studies addressing the association between maternal BMI and risk of ASD in offspring were included. We used random-effect models to estimate the summary relative risks (RRs), we also performed a dose-response meta-analysis to estimate the trend from the correlated log RR estimates across levels of BMI quantitatively. Totally, 6 cohort studies and 1 case-control study involving 8,403 cases and 509,167 participants were included for analysis. The summary RR (95% confidence interval) for ASD in offspring in relation to maternal underweight, overweight, and obesity vs. normal weight during pre-pregnancy or pregnancy, was 1.07 (0.93, 1.23), 1.28 (1.19, 1.36) and 1.36 (1.03, 1.78), respectively. A linear dose-response relationship was found, with a pooled RR of 1.16 (1.01, 1.33) for each 5 kg/m2. increment in maternal BMI. The present study suggests that excessive maternal BMI is associated with increased ASD risk in offspring.
Similar content being viewed by others
Introduction
Autism spectrum disorders (ASD) are a group of complex neurodevelopmental disorders characterized by impairments in social interaction and communications, as well as restricted and repetitive behaviors1. The reported prevalence of ASD has been increasing since the 1990 s, and it is projected to be 1 person in 132 in 2010 worldwide2. While the etiology of ASD still remains unclear, both genetic and environmental factors are thought to play a role3. Among diverse factors, maternal conditions during pre-pregnancy or pregnancy are increasingly being recognized as potential risk factors for ASD. Evidence from meta-analysis indicated that maternal advanced age4, diabetes5, and antidepressants use6 during pregnancy was associated with elevated ASD risk in offspring. Maternal obesity, which has become a global health problem, has also been studied by several epidemiological studies to examine a possible association with ASD. However, the results were inconclusive. For example, while the study by Xaing et al.7 and Garndner et al.8 found a positive association between maternal obesity and ASD risk, the study by Moss et al.9 found no statistically significant results.
A recent meta-analysis by Li et al.10 reviewed the studies regarding maternal obesity and ASD risk and found a positive relationship of maternal obesity with ASD risk. Nevertheless, they included one study using body weight as cut-off point for obesity which might introduce bias because other included studies ascertain obesity based on body mass index (BMI). Moreover, Li et al.10 only focused on the effect of maternal obesity on ASD in offspring, but failed to fully assess the potential association of different category of BMI including overweight and underweight with ASD risk, and they failed to explore a possible dose-response relation of BMI and ASD risk because of the insufficient data. In this study, we aimed to systematically assess the association between maternal BMI (underweight, overweight and obesity) and ASD risk in offspring, trying to find a dose-response relation between them.
Results
Literature search and selection
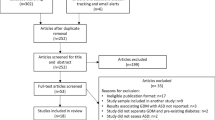
We retrieved 1525 records using the search strategy. After removal of duplicate literatures, 1123 articles were left for screening. From screening the titles and abstracts, 1111 articles were excluded as they were not clearly relevant. After evaluating the full text of the remaining 12 literatures, we further excluded 5 studies11,12,13,14,15 that did not report risk estimates of concern. Finally, 7 studies7,8,9,14,16,17,18 were included for analysis (See Supplemental Figure 1).
Study characteristics
Table 1 lists the characteristics of the included studies, which consisted of 6 cohort studies (5 prospective and 1 retrospective) and 1 case-control studies. There were 8,403 cases and 509,167 participants. While 5 studies were conducted in the US, 2 studies were from Europe (Norway and Sweden). The information about BMI was generally collected during pre-pregnancy, and ASDs were ascertained by standard assessment in most of studies. The methodological quality of all studies was good, with a range score of 7–9 (average: 8.1) (See Supplemental Table 1).
Categorical meta-analysis
Compared to children born to mothers with normal weight (or non-obese weight), the pooled RR (95% confidence interval, CI) of ASD was 1.07 (0.93, 1.23), 1.28 (1.19, 1.36) and 1.36 (1.03, 1.78) for children born to mothers with underweight, overweight and obesity, with low heterogeneity (I2 = 0.0%, P = 0.929), low heterogeneity (I2 = 0.0%, P = 0.521) and high heterogeneity (I2 = 77.5%, P < 0.001) observed across studies, respectively (Figs 1,2 and 3).
Dose-response meta-analysis
4 studies were included for dose-response analysis. A linear dose-response relationship was found between maternal BMI and risk of ASD (P-nonlinearity = 0.673). Compared with children born to mothers in normal weight, the pooled RR for ASD in offspring was 1.16 (1.01, 1.33) for each 5 kg/m2. increment in maternal BMI (Fig. 4).
Subgroup and sensitivity analysis
Table 2 summarizes the main findings of the meta-analysis according to categories of maternal BMI versus normal weight stratified by study design, study location, and adjustment for confounders. For children born to underweight mothers, the summary risk estimates for ASD were generally comparable and similar to that of overall result when stratified by different factors. Stronger associations between maternal overweight/obesity and ASD risk were found in European studies, and in which BMI were measured at first trimester and ASD were ascertained by standard assessment. When stratified by certain confounders, stronger associations were found in studies adjusted for maternal age, child sex, and birth year than studies that did not adjust for such confounders. In sensitivity analysis, the summary RRs did not substantially changed for different categories of maternal BMI before and after elimination of each study in the meta-analyses, which indicated that our results were robust.
Publication bias
There was no evidence of significant publication bias among the included studies according to Begg’s test and Egger’s test (all P > 0.05).
Discussion
In this meta-analysis of observational studies, we explored the effect of maternal BMI (underweight, overweight or obesity) during pre-pregnancy or pregnancy on ASD risk in offspring. Compared with children whose mothers were at normal weight, children born to overweight and obese mothers have a 28% and 36% higher risk of developing ASD, respectively. Maternal underweight was not associated with increased ASD risk. A linear dose-response relationship was found, with the risk of ASD increasing by 16% for each 5 kg/m2 increment in maternal BMI compared with that of normal weight. ASD is one of the most common and severe neurodevelopmental disorders which is lifelong. It not only significantly impacts upon the individuals, but also has long-term implications for their families, as well as for the provision of education and habilitative services19. Some behavioral treatments have been suggested to produce positive short-term benefits19. Nevertheless, given that the noticeable clinical and genetic heterogeneity between affected individuals, the lack of reliable diagnostic biomarkers, and the unrevealed underlying pathophysiological mechanisms, there are still no effective treatments for the core symptoms of ASD20. It is therefore crucial to identify related risk factors and to prevent ASD in the primary step.
Along with the nearly doubled world’s obesity rate between 1980 and 2008, the prevalence of ASD has also been increasing rapidly during the same period. While elevated awareness and updated diagnostic criteria of ASD might contribute to its increased prevalence, it is possible that the obesity epidemic may also play a role, which is supported by the results of our meta-analysis. The present findings provide strong persuasion for women to keep appropriate weight during pre-pregnancy or pregnancy, so as to reduce ASD risk in their offspring.
Although the causal pathway remains to be elucidated, the effects of maternal BMI on ASD may be explained by several hypothesizes. Of the proposed mechanisms, inflammation is the most frequently mentioned one to explain the association of maternal BMI and ASD. It is observed that obese women had higher levels of C-reactive protein in the plasma compared with normal weight pregnant women21. In addition, increased CD68 + and CD14 + cells with elevated expression of inflammatory cytokines including tumor necrosis factor-alpha, interleukin-6, and interleukin-1 in the placentas have also been found in obese pregnant mothers22,23. As placenta inflammation is associated with neonatal brain damage and can induce a systemic fetal inflammatory response which may contribute to white matter injury in the fetal brain23, it is plausible that the risk of mental disorders increases for children born to mother with overweight/obesity.
Obesity is a significant risk factor for diabetes, while maternal diabetes itself significantly increase the risk of ASD in the offspring5. Hyperglycemia, as a consequence of maternal diabetes, is supposed to increase ASD risk in offspring through several mechanisms, such as hypoxia in the fetus, increased free-radical production and impaired antioxidant defense system24,25,26. Dietary and nutrition factors plays important role in the development of both obesity and diabetes. However, nutritional factors such as fat intake, vitamins may also contribute to the development of ASD27,28. Nevertheless, the association between maternal obesity and ASD risk in offspring may be simultaneously mediated by multiple factors and the explicit mechanism needs further elucidation.
This meta-analysis was based on observational studies. Therefore, we cannot exclude potential biases due to other factors which may contribute to ASD. For instance, mothers who have lower BMI will probably have more healthy lifestyles, such as more physical exercise, less intake of salt and saturated fat, and thus they are less likely to be affected by hyperglycemia and diabetes than those who have higher BMI. Although all studies controlled for several factors, including maternal age at baseline, race, child’s sex, and birth year etc, the potential influences from residual or unmeasured confounding cannot be ruled out.
Apart from maternal BMI, paternal obesity was also suggested to be associated with risk of autism in offspring. In two included studies, Gardner et al.8 and Surén et al.18 respectively reported a 47% (OR 1.47, 95% CI 1.12–1.92) and 73% (OR 1.73, 95% CI 1.07–2.82) increased risk of fathers with obesity having a child diagnosed with autism, compared with the risk of autism in children of nonobese fathers. One reason of this association might be obesity-related alterations to epigenetic information, which modulate gene expression that is essential for proper development after fertilization29. Moreover, the two studies also suggested that when adjusted for paternal and other confounding factors, the risk of autism in children with obese mothers was attenuated. Considering that paternal obesity was not adjusted in other five included studies, the positive association between maternal BMI and risk of autism concluded by this meta-analysis might be overestimated.
Two recent studies8,12 found that the association between maternal BMI and offspring risk of ASD does not hold when ASD cases were compared to their matched, unaffected siblings. Both the sibling study and the study of paternal BMI suggested that maternal BMI might be a proxy marker for inherited background which potentially explains some of the risk attributable to maternal BMI8. An evidence is that the deletion or duplication in a region of chromosome 16p11.2 was indicated to influence susceptibility to autism30, meanwhile 16p11.2 deletions were associated with obesity and morbid obesity at or near genome-wide levels of significance, compared to lean/normal weight subjects31. Therefore, the potential underlying genetic mechanism regarding maternal BMI and risk of ASD in offspring should be further explored in future studies.
Maternal BMI were self-reported without objective ascertainment in 3 included studies9,17,18. Nevertheless, women tend to overestimate their height and underestimate their weight by self-report32,33. There was possibility that the risk of ASD in offspring was underestimated as the BMI might be underestimated. In addition, six studies7,9,14,16,17,18 measured maternal BMI at pre-pregnancy while one8 assessed it at the first antenatal visit, lacking of a continuous documentation. However, the process of pregnancy often accompanies with weight gain34. While excessive prenatal weight gain is suggested to be associated with elevated risk of ASD in offspring12, the positive association between maternal BMI and ASD risk might partially be attributed to excessive prenatal weight gain. Nevertheless, besides Gardner et al.8 adjusted for gestational weight gain in one of their sensitivity analysis, none of other included studies adjusted for weight gain during pregnancy. It is therefore necessary for future studies to exclude the possible effect of prenatal weight gain on ASD risk.
Our meta-analysis included 2 retrospective studies7,16. Retrospective design may incur several biases, including selection bias and recall bias. The improper selection of cases and controls, the biased collection of exposure and related confounding factors, can both lead to a biased result. Nevertheless, when we restricted study design to prospective cohort studies, the results did not markedly alter, suggesting that study design did not significantly influence the general results.
In conclusion, the present study suggests that excessive maternal BMI is associated with an increased ASD risk in offspring. Approximately, every 5 kg/m2 increment in BMI is associated with 16% increased ASD risk. Considering the limited small number of included studies, further well-designed studies are needed to confirm the our findings.
Methods
Data sources and searches
We conducted a literature search of PubMed, Embase, Web of Science, and Cochrane Library up to 29 January 2016 for case-control and cohort studies examining the association between maternal BMI and risk of ASD in offspring. The search terms for PubMed were: (body mass index OR overweight OR obesity) AND autism. Similar search strategies were used in other databases. In addition, we screened references list from relevant original papers and review articles to identify further pertinent studies. We applied no language restriction in the process of literature search and selection. Two researchers independently conducted the literature search and disagreement was solved by discussion. The Meta-Analysis of Observational Studies in Epidemiology guidelines35 was followed to conduct this meta-analysis.
Study selection
Studies were included in the meta-analyses if they were case-control or cohort studies which reported the association between maternal BMI and risk of ASD in offspring. To be included, studies had to have available results as an odds ratio (OR) or a relative risk (RR) with corresponding 95% confidence interval (CI), or sufficient data to calculate them. Non-human studies, case reports, conference abstract, review and studies that with insufficient data were excluded.
Data extraction and quality assessment
The following data were extracted from each study: name of the first author, publication year, study name and period, study location, characteristics of participants (sex and age), number of cases and participants, the RRs or ORs with corresponding 95% CIs, confounding factors that being adjusted for in the analysis. We also extracted dose-relationship data for maternal BMI and ASDs risk, including the number of cases and participants (or person-years) and RR (95% CI) for each category of BMI.
The Newcastle-Ottawa Scale (NOS)36, which has been widely used for quality assessment of observational studies, was used to assess the methodological quality of included studies. This scale awards a maximum of nine points to each study: selection of the study groups (maximum 4 points), comparability of the study populations (maximum 2 points) and ascertainment of the outcome of interest (maximum 3 points). Studies that scored 0–3, 4–6, and 7–9 were considered as low, moderate, and high quality, respectively.
Data analysis
To take into account heterogeneity between studies, a random-effects model was used to calculate summary RRs and 95%CI for non-reference (obese, overweight and underweight) versus the reference (normal weight) categories of BMI and for the dose-response analysis. The odds ratios (ORs) were considered equivalent to RRs considering that ASDs were sufficiently rare. Heterogeneity between studies was assessed by using Q statistics (significance level of P  0.10) and I2 statistics37,38. I2 values of 25%, 50%, and 75%, was considered to be low, moderate, and high degrees of heterogeneity, respectively38. Subgroup analyses were conducted across a number of key study characteristics, including study location (Europe, USA), study design (prospective or retrospective), time of BMI measurement (pre-pregnancy, pregnancy), ascertainment of ASD (parental report, standard assessment), adjustment for maternal age (yes, no), child sex (yes, no), and birth year. We also performed sensitivity analysis to explore the effect of each individual study on the pooled result. Potential publication bias was examined by the Begg’s test39 and Egger’s test40.
0.10) and I2 statistics37,38. I2 values of 25%, 50%, and 75%, was considered to be low, moderate, and high degrees of heterogeneity, respectively38. Subgroup analyses were conducted across a number of key study characteristics, including study location (Europe, USA), study design (prospective or retrospective), time of BMI measurement (pre-pregnancy, pregnancy), ascertainment of ASD (parental report, standard assessment), adjustment for maternal age (yes, no), child sex (yes, no), and birth year. We also performed sensitivity analysis to explore the effect of each individual study on the pooled result. Potential publication bias was examined by the Begg’s test39 and Egger’s test40.
For dose-response analysis, the method proposed by Greenland and Longnecker41 and Orsini et al.42 was used to compute study-specific slopes (linear trends) and 95% CIs from the natural logs of the RRs and CIs across categories of BMI. This method requires that the numbers of ASDs and persons/person-years for at least three BMI categories, and means or medians of the categories had to be available. When the median or mean BMI per category was not reported, the midpoint of the upper and lower boundaries was considered the BMI of each category. If the lower or upper boundaries for the lowest and highest category were not available, we assumed the length of these categories to be the same as the closest category. We used centered dose levels (each original non-reference dose minus the reference dose within a study) to summarize dose-response relation43.
A 2-stage random-effects dose-response meta-analysis was used to examine the potential trend between maternal BMI and risk of ASD42,44. We first applied restricted cubic splines with three knots in settled percentiles (10%, 50%, and 90%) of the distribution to model the possible association44. Then we derived the overall estimates with random effects by pooling the study-specific coefficient estimates and variance/covariance matrices that had been obtained in the first stage45. We calculated a P-value for nonlinearity by testing the hypothesis that the coefficient of the second spline was different from zero. For the study included in the dose-response analysis, we estimated a RR and corresponding 95% CIs for a 5 kg/m2 increase from 18 to 38 kg/m2 in BMI. STATA version 12.0 (StataCorp LP, College Station, TX) was used to analyze the data, and the significance level was set as 0.05 where otherwise specified.
Additional Information
How to cite this article: Wang, Y. et al. Maternal Body Mass Index and Risk of Autism Spectrum Disorders in Offspring: A Meta-analysis. Sci. Rep. 6, 34248; doi: 10.1038/srep34248 (2016).
References
Lai, M. C., Lombardo, M. V. & Baron-Cohen, S. Autism. Lancet (London, England) 383, 896–910, 10.1016/s0140-6736(13)61539-1 (2014).
Baxter, A. J. et al. The epidemiology and global burden of autism spectrum disorders. Psychological medicine 45, 601–613, 10.1017/s003329171400172x (2015).
Duchan, E. & Patel, D. R. Epidemiology of autism spectrum disorders. Pediatric clinics of North America 59, 27–43, ix-x, 10.1016/j.pcl.2011.10.003 (2012).
Sandin, S. et al. Advancing maternal age is associated with increasing risk for autism: a review and meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry 51, 477–486 e471, 10.1016/j.jaac.2012.02.018 (2012).
Xu, G., Jing, J., Bowers, K., Liu, B. & Bao, W. Maternal diabetes and the risk of autism spectrum disorders in the offspring: a systematic review and meta-analysis. Journal of autism and developmental disorders 44, 766–775, 10.1007/s10803-013-1928-2 (2014).
Rais, T. B. & Rais, A. Association Between Antidepressants Use During Pregnancy and Autistic Spectrum Disorders: A Meta-analysis. Innovations in clinical neuroscience 11, 18–22 (2014).
Xiang, A. H. et al. Association of maternal diabetes with autism in offspring. Jama 313, 1425–1434, 10.1001/jama.2015.2707 (2015).
Gardner, R. M. et al. Maternal body mass index during early pregnancy, gestational weight gain, and risk of autism spectrum disorders: Results from a Swedish total population and discordant sibling study. International journal of epidemiology 44, 870–883, 10.1093/ije/dyv081 (2015).
Moss, B. G. & Chugani, D. C. Increased risk of very low birth weight, rapid postnatal growth, and autism in underweight and obese mothers. American journal of health promotion: AJHP 28, 181–188, 10.4278/ajhp.120705-QUAN-325 (2014).
Li, Y. M. et al. Association Between Maternal Obesity and Autism Spectrum Disorder in Offspring: A Meta-analysis. Journal of autism and developmental disorders 46, 95–102, 10.1007/s10803-015-2549-8 (2016).
Antoniou, E. E. et al. Maternal pre-pregnancy weight and externalising behaviour problems in preschool children: a UK-based twin study. BMJ open 4, e005974, 10.1136/bmjopen-2014-005974 (2014).
Bilder, D. A. et al. Maternal prenatal weight gain and autism spectrum disorders. Pediatrics 132, e1276–e1283, 10.1542/peds.2013-1188 (2013).
Jo, H. et al. Maternal prepregnancy body mass index and child psychosocial development at 6 years of age. Pediatrics 135, e1198–e1209, 10.1542/peds.2014-3058 (2015).
Reynolds, L. C., Inder, T. E., Neil, J. J., Pineda, R. G. & Rogers, C. E. Maternal obesity and increased risk for autism and developmental delay among very preterm infants. Journal of perinatology: official journal of the California Perinatal Association 34, 688–692, 10.1038/jp.2014.80 (2014).
Tanne, J. H. Maternal obesity and diabetes are linked to children’s autism and similar disorders. BMJ (Clinical research ed.) 344, e2768, 10.1136/bmj.e2768 (2012).
Krakowiak, P. et al. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics 129, e1121–e1128, 10.1542/peds.2011-2583 (2012).
Lyall, K., Pauls, D. L., Santangelo, S. L., Spiegelman, D. & Ascherio, A. Maternal early life factors associated with hormone levels and the risk of having a child with an autism spectrum disorder in the nurses health study II. Journal of autism and developmental disorders 41, 618–627, 10.1007/s10803-010-1079-7 (2011).
Suren, P. et al. Parental obesity and risk of autism spectrum disorder. Pediatrics 133, e1128–e1138, 10.1542/peds.2013-3664 (2014).
Vismara, L. A. & Rogers, S. J. Behavioral treatments in autism spectrum disorder: what do we know? Annual review of clinical psychology 6, 447–468, 10.1146/annurev.clinpsy.121208.131151 (2010).
Loth, E., Spooren, W. & Murphy, D. G. New treatment targets for autism spectrum disorders: EU-AIMS. The lancet. Psychiatry 1, 413–415, 10.1016/s2215-0366(14)00004-2 (2014).
Madan, J. C. et al. Maternal obesity and markers of inflammation in pregnancy. Cytokine 47, 61–64, 10.1016/j.cyto.2009.05.004 (2009).
Challier, J. C. et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta 29, 274–281, 10.1016/j.placenta.2007.12.010 (2008).
van der Burg, J. W. et al. The role of systemic inflammation linking maternal BMI to neurodevelopment in children. Pediatric research 79, 3–12, 10.1038/pr.2015.179 (2016).
Biri, A. et al. Oxidant status in maternal and cord plasma and placental tissue in gestational diabetes. Placenta 27, 327–332, 10.1016/j.placenta.2005.01.002 (2006).
Chen, X. & Scholl, T. O. Oxidative stress: changes in pregnancy and with gestational diabetes mellitus. Current diabetes reports 5, 282–288 (2005).
Eidelman, A. I. & Samueloff, A. The pathophysiology of the fetus of the diabetic mother. Seminars in perinatology 26, 232–236 (2002).
Lyall, K., Munger, K. L., O’Reilly, E. J., Santangelo, S. L. & Ascherio, A. Maternal dietary fat intake in association with autism spectrum disorders. American journal of epidemiology 178, 209–220, 10.1093/aje/kws433 (2013).
Lyall, K., Schmidt, R. J. & Hertz-Picciotto, I. Maternal lifestyle and environmental risk factors for autism spectrum disorders. International journal of epidemiology 43, 443–464, 10.1093/ije/dyt282 (2014).
Murphy, S. K. Obesity: Paternal obesity–a risk factor for autism? Nature reviews. Endocrinology 10, 389–390, 10.1038/nrendo.2014.81 (2014).
Weiss, L. A. et al. Association between microdeletion and microduplication at 16p11.2 and autism. The New England journal of medicine 358, 667–675, 10.1056/NEJMoa075974 (2008).
Jacquemont, S. et al. Mirror extreme BMI phenotypes associated with gene dosage at the chromosome 16p11.2 locus. Nature 478, 97–102, 10.1038/nature10406 (2011).
Gunnare, N. A., Silliman, K. & Morris, M. N. Accuracy of self-reported weight and role of gender, body mass index, weight satisfaction, weighing behavior, and physical activity among rural college students. Body image 10, 406–410, 10.1016/j.bodyim.2013.01.006 (2013).
Tsai, E. W. et al. Accuracy of self-reported weight and height in women from Bogota, Colombia. Annals of human biology 41, 473–476, 10.3109/03014460.2013.856939 (2014).
Smith, D. E. et al. Longitudinal changes in adiposity associated with pregnancy. The CARDIA Study. Coronary Artery Risk Development in Young Adults Study. Jama 271, 1747–1751 (1994).
Stroup, D. F. et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama 283, 2008–2012 (2000).
Wells, G. et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. Ottawa, Ontario: The Ottawa Health Research Institute. (2011).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Statistics in medicine 21, 1539–1558, 10.1002/sim.1186 (2002).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed.) 327, 557–560, 10.1136/bmj.327.7414.557 (2003).
Begg, C. B. & Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 (1994).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed.) 315, 629–634 (1997).
Greenland, S. & Longnecker, M. P. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. American journal of epidemiology 135, 1301–1309 (1992).
Orsini, N., Bellocco, R. & Greenland, S. Generalized least squares for trend estimation of summarized dose-response data. Stata Journal 6, 40 (2006).
Liu, Q., Cook, N. R., Bergström, A. & Hsieh, C.-C. A two-stage hierarchical regression model for meta-analysis of epidemiologic nonlinear dose–response data. Computational Statistics & Data Analysis 53, 4157–4167 (2009).
Orsini, N., Li, R., Wolk, A., Khudyakov, P. & Spiegelman, D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. American journal of epidemiology 175, 66–73, 10.1093/aje/kwr265 (2012).
Jackson, D., White, I. R. & Thompson, S. G. Extending DerSimonian and Laird’s methodology to perform multivariate random effects meta-analyses. Statistics in medicine 29, 1282–1297, 10.1002/sim.3602 (2010).
Acknowledgements
The authors would like to thank Prof. Qiqiang He (Wuhan University, Wuhan, China) for his help in language improvement. This work was supported by the National Natural Science Foundation of China [30971040, 81271496], the National Key Technology R&D Program during the 12th Five-Year of China [2012BAI01B05], and the Fundamental Research Funds for the Central Universities [2042014kf0273].
Author information
Authors and Affiliations
Contributions
Z.L. conceived and designed the experiments; Y.W., S.T. and S.X. performed the experiments; Y.W., S.T. and S.W. analyzed the data; S.X. and S.W. contributed reagents/materials/analysis tools; Z.L., Y.W. and S.T. wrote the paper. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wang, Y., Tang, S., Xu, S. et al. Maternal Body Mass Index and Risk of Autism Spectrum Disorders in Offspring: A Meta-analysis. Sci Rep 6, 34248 (2016). https://doi.org/10.1038/srep34248
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep34248
This article is cited by
-
Autism spectrum disorders and the gastrointestinal tract: insights into mechanisms and clinical relevance
Nature Reviews Gastroenterology & Hepatology (2024)
-
Is the association between mothers’ autistic traits and childhood autistic traits moderated by maternal pre-pregnancy body mass index?
Molecular Autism (2023)
-
Maternal Exposure to Pesticides and Risk of Autism Spectrum Disorders in Offspring: A Meta-analysis
Journal of Autism and Developmental Disorders (2022)
-
Maternal acute and chronic inflammation in pregnancy is associated with common neurodevelopmental disorders: a systematic review
Translational Psychiatry (2021)
-
Maternal immune activation and neuroinflammation in human neurodevelopmental disorders
Nature Reviews Neurology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.