Abstract
Inherited monogenic diseases of the retina and vitreous affect approximately 1 in 2000 individuals. They are characterized by tremendous genetic heterogeneity and clinical variability involving mutations in approximately 250 genes and more than 20 different clinical phenotypes. Clinical manifestations of retinal dystrophies (RDs) range from mild retinal dysfunctions to severe congenital forms of blindness. A detailed clinical diagnosis and the identification of causative mutations are crucial for genetic counseling of affected patients and their families, for understanding genotype-phenotype correlations and developing therapeutic approaches. Using whole exome sequencing (WES) we have established a reliable and efficient high-throughput analysis pipeline to identify disease-causing mutations. Our data indicate that this approach enables us to genetically diagnose approximately 64% of the patients (n = 58) with variant(s) in known disease-associated genes. We report 20 novel and 26 recurrent variants in genes associated with RDs. We also identified a novel phenotype for mutations in C2orf71 and provide functional evidence for exon skipping due to a splice-site variant identified in FLVCR1. In conclusion, WES can rapidly identify variants in various families affected with different forms of RDs. Our study also expands the clinical and allelic spectrum of genes associated with RDs in the Swiss population.
Similar content being viewed by others
Introduction
Monogenic retinal dystrophies (RDs) account for vision loss in more than 2 million people worldwide1. The genetic basis of RDs is extremely heterogeneous. Mutations in approximately 250 genes are currently known to be responsible for over 20 different clinically distinguished phenotypes. Clinical manifestations for these disorders range from mild dysfunctions (such as night blindness) to severe and early onset RDs (such as Leber congenital amaurosis, LCA). Retinitis pigmentosa (RP) is the most common form of familial RDs affecting 1 in 3500–4000 people. It affects predominantly the rod photoreceptors and can be transmitted in an autosomal recessive (arRP), dominant (adRP) or an X-linked mode of inheritance (XlRP)1,2. arRP is considered more severe in terms of a younger age of onset and faster disease progression and accounts for over 50–60% of all RP cases. adRP usually is less severe, accounts for 30–40% of RP cases and is associated with a later age of onset1,2. In addition, incomplete penetrance has been described for a number of dominant gene mutations3,4,5,6. XlRP is seen in 5–15% of RP cases2. Clinical manifestations in RP typically begin with night blindness. Later, during disease progression, tunnel vision is characteristic and the disease may in some cases lead to complete blindness. Cone dystrophies (COD) and cone-rod dystrophies (CORD) are more severe compared to rod-dominated phenotypes1. The age of onset is usually during early adult life. So far there are 33 genes known to be involved in COD or CORD (RetNet: https://sph.uth.edu/retnet/home.htm). Mutations in the respective genes are autosomal dominant or recessive as well as X-linked. LCA is one of the most severe forms of RDs involving rod and cone photoreceptor cells. Patients usually present at birth with severe vision loss and nystagmus1. Currently, mutations in 23 genes are known to cause LCA (RetNet). The frequency of the disease is about 1 in 50,000. Stargardt disease (STGD) is one of the most frequent autosomal recessive macular dystrophy in childhood or adolescence with an estimated incidence of 1 in 10,000. Mutations in 4 genes have been reported to cause STGD, with mutations in ABCA4 gene being the most frequent. Best macular dystrophy (Best MD) is a stationary or slowly progressing form of macular dystrophy with a variable age of onset between childhood and late teenage years. Best MD (due to mutations in BEST1) is most commonly inherited in an autosomal dominant manner but autosomal recessive forms have also been reported7. In addition, RDs can be either non-syndromic or syndromic (in combination with non-ocular symptoms). Some of the common syndromic forms are characterized by hearing loss (e.g., Usher syndrome), polydactyly (e.g., Bardet-Biedl syndrome, BBS), or renal abnormalities (e.g., Senior–Løken syndrome).
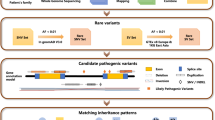
The identification of disease-causing mutations has been tremendously accelerated by implementation of next generation sequencing (NGS) technologies in diagnostic genetic testing in familial cases of RDs but also in sporadic patients. In this study, we employed whole exome sequencing (WES) in 58 patients with 11 clinically different RDs. We were able to identify putative disease causing variants and thus provide a genetic diagnosis in 64% of the cases. The identified mutation spectrum included novel variants and previously described recurrent mutations. Cases and families, where underlying genetic mutations were not identified, might be instrumental for the identification of novel disease-associated genes.
Results
Patients
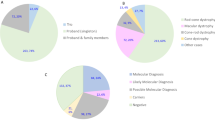
The majority of analyzed patients (n = 58) originated from Switzerland. We cannot however exclude that some patients have migrated to Switzerland (24.3% foreigners in Switzerland; Source: http://www.bfs.admin.ch/). All of the patients were clinically well-defined and included RP (n = 31), STGD (n = 5), LCA (n = 5), CORD (n = 3), RD not classified further (n = 3), COD (n = 3), Macular dystrophy (n = 3), Best disease (n = 2), Choroideremia (n = 1), BBS (n = 1) and Usher syndrome (n = 1). 38 of these were individual cases with no samples from additional family members. For the remaining 20 cases, samples from additional family members were available to perform segregation analysis.
Whole exome sequencing
HiSeq2000 data: 34 cases were sequenced on the HiSeq2000. 7.2–9.3 Gb of data were obtained upon WES that constituted about 9.0*107 reads, with >30 coverage ranging from 81–92% within the different sequences. 550–600 variants were obtained within the known RD genes (n = 252) and an average of approximately 40,000 variants were obtained with WES. Most of the variants identified were synonymous, closely followed by missense variants. A large percentage of the variants lie within the captured intronic regions flanking the exons (Fig. 1a, Supplementary Figure 1). A large number of variants obtained were predicted to affect splicing by either strongly or weakly activating splice acceptor or donor sites.
NextSeq500 data: 24 cases were sequenced on the NextSeq500. Approximately 12 Gb of data were obtained upon WES and 7–9*107 reads, with >20 coverage ranging from 88–93% within the different sequences. The type of variants obtained on the NextSeq500 showed a similar trend as obtained on the HiSeq2000. However, the number of variants obtained was higher with NextSeq500 compared to that of HiSeq2000.
Figure 1a compares the types and numbers of variants obtained within the 252 genes upon sequencing with NextSeq500 versus HiSeq2000. Supplementary Figure 1 compares the type and number of variants obtained within the entire exome data.
Sequence variants identified in RD cases
We performed WES in 58 cases and identified potentially disease-causing variants in known RD genes in 37 (64%) index patients, 25 of whom were simplex/sporadic cases. For 13 cases we had samples from additional family members. Figure 1b shows a comparison of the mutation detection rates in various disease phenotypes. A total of 65 mutant alleles were identified in known RD genes. While 37 (57%) of these have been previously associated with RD, 28 (43%) are novel variants in known RD genes. Amongst the identified disease-associated variants, 24 (36.9%) are missense variants, 18 (27.7%) are nonsense variants, 16 (24.6%) are frameshift deletions or insertions and 7 (10.8%) are predicted to affect splicing (Fig. 2a). In our cohort, the majority of variants were identified in ABCA4 (21.54%), followed by C2orf71 (15.38%) and RP1 (7.69%) and then CEP290, FLVCR1 and CRB1 each contributing 6.15% to the total variants. Variants were also identified in 12 additional genes (Fig. 2b)
ABCA4
Mutations in ABCA4 have been previously associated with STGD, RP, MD, COD or CORD phenotypes1,8,9. In our patient cohort, the majority of variants were identified in ABCA4 (21.54%) (Fig. 2b), which included 1 RP, 1 RD, 1 MD, 2 COD and 2 STGD cases (n = 7) (Table 1, Fig. 3a,b). We identified 14 mutant alleles in ABCA4, including 2 novel variants. In case 71674, diagnosed with arRD, along with a recurrent nonsense mutation (p.Trp663*, HGMD Accession = CM003370), a novel splice-site variant was identified (c.2160 + 1 G > T) (Fig. 3a). This variant is predicted to lead to skipping of exon 14 in the ABCA4 transcript. The patient inherited one mutant allele from each parent. A novel missense variant was identified in case 71472 diagnosed with RP (Table 1).
C2orf71
Mutations in C2orf71 are known to cause arRP and were first reported by Collen et al. in 201010. Within our cohort, mutations in C2orf71 accounted for the second highest number of mutations (15.38%). We identified potentially pathogenic variants in C2orf71 in four patients affected with arRP and one diagnosed with CORD (Table 1). Almost 50% of the mutations in HGMD described in C2orf71 are truncating mutations. Consistently, all the mutations we identified in our patient cohort in C2orf71 are also truncating mutations including three novel variants (p.Trp650*, p.Gly570Glufs*3, p.Leu744Glufs*7). Index patient 29870 and his affected brother were diagnosed with RP (Fig. 4a) and carried a homozygous frameshift deletion in C2orf71: NM_001029883.2: c.1709_1728del:p.Gly570Glufs*3. This mutation was absent in the unaffected brother. Interestingly, we also identified the mutation p.Gly570Glufs*3 in two other cases (71703 and 71688) in a compound heterozygous state with two additional mutations (Table 1). So far mutations in C2orf71 have been described exclusively for RP. We present a novel phenotype for mutations in this gene, leading to cone-rod dystrophy (Case 71703). The affected sibling of index 71703 was also diagnosed with CORD and carries the same mutations (data not shown). No additional samples from family members were available to perform segregation analysis.
RP1
Mutations in RP1 are known to cause RP and are inherited in autosomal recessive or dominant manner. We identified 2 previously described mutations in 4 patients diagnosed with RP (Table 1): a homozygous nonsense mutation (p.Ser542*, HGMD Accession = CM1211361) in an arRP case; and a heterozygous duplication leading to a frameshift (p.Arg872Thrfs*2, HGMD Accession = CI004598), which was identified in three patients diagnosed with adRP (Table 1). Index patient 24058 has one affected brother and one unaffected sister. They inherited the mutation from their father who had 3 affected brothers and an affected father (Fig. 4b). We also identified this mutation in three additional families, which were not part of this study (data not shown). The rather high frequency of this mutation in our cohort suggests it to be more abundant in the Swiss population. The mutation was absent in 528 control alleles.
CEP290
We identified compound heterozygous mutations in CEP290 in two cases. Index patient 30421 was diagnosed with LCA, while his parents were unaffected (Fig. 3g). A known frameshift deletion NM_025114.3:c.6604del:p.Ile2202Leufs* 24 (HGMD Accession = CD072355) was found in 30421. Index patient 13730 was diagnosed with arRP and has two affected and two unaffected siblings (Fig. 4c). Using WES, a previously described nonsense mutation was identified in 13730: NM_025114.3:c.5668G>T:p.G1890* (HGMD Accession = CM061683). In both cases, the mutations were heterozygous and a mutation in the second allele was missing. Since the patients were diagnosed with classical recessive diseases, we wondered if they carried a common deep intronic mutation in CEP290. This mutation NM_025114.3:c.2991+1655A>G (HGMD Accession = CS064383) accounts for 21–43% of mutations in LCA patients11,12. It leads to an activation of a cryptic splice donor site and inserts a cryptic exon in the messenger RNA of CEP29011,12. Upon Sanger sequencing, we were able to confirm this mutation in patients 30421 and 13730. Analyzing the family members, we confirmed that patient 30421 inherited one mutant allele from each parent (Fig. 3g). Patient 13730 and her affected brother also carried both the mutations, while her unaffected mother was a carrier of the intronic mutation c.2991 + 1655 A > G (Fig. 4c). It can be assumed that the affected members inherited the nonsense mutation in CEP290 from the deceased father, samples from whom were not available.
FLVCR1
We found a novel missense variant in FLVCR1 and a previously described splice-site variant in 2 patients diagnosed with RP: c.479 T > C (p.Leu160Pro) and c.1092 + 5 G > A (Table 1). The missense variant affects a highly conserved amino acid and is classified to be disease-causing by 3 different prediction tools (SIFT, PolyPhen2, MutationTaster2). The splice-site variant lies within the consensus splice donor site13 and therefore is predicted to affect the normal splicing of exon 4 (Fig. 5a). While case 71315 was compound heterozygous for the above variants, case 29303 is homozygous for the c.1092 + 5 G > A variant. Both patients inherited one mutant allele respectively from each unaffected parent (Figs 3d,e and 5b,c).
(a) Partial gene structure of FLVCR1 is displayed in a schematic drawing. Position of the mutation is indicated with an arrow. This mutation is predicted to affect the canonical splice donor site of exon 4 in FLVCR1. (b) Sanger sequence of patient DNA showing homozygous mutation c.1092 + 5G > A in FLVCR1. (c) Sanger sequence of DNA from patient’s mother showing heterozygous mutation c.1092 + 5G > A in FLVCR1. Father of the patient was also heterozygous (data not shown). (d) Agarose gel image of RT-PCR products from blood RNA. An unrelated, and unaffected individual was used as control. While patient and control both show a product of expected size, the patient sample shows an additional shorter band indicating skipping of exon 4. This was verified by sequencing of both products. (e) Alignment of FLVCR1 cDNA sequence obtained from the patient in comparison to reference showing the skipping of exon 4 (highlighted in yellow). (f) Alignment of the FLVCR1 cDNA sequence from the control sample in comparison to reference showing no skipping of exon 4.
Analysis of FLVCR1 splicing
The effect of the variant (c.1092 + 5 G > A) on the correct splicing of FLVCR1 has not been studied previously. We extracted total RNA from venous blood of patient 29303 and performed reverse transcription-PCR (RT-PCR). We analyzed splicing of exons 1 through 7 and detected two isoforms exclusively in the patient: one PCR product of expected size and a second shorter product (Fig. 5d). Upon sequencing of these products, we confirmed that the shorter product lacked exon 4 (Fig. 5e). Exon 4 skipping is highlighted in yellow in the sequence alignment of FLVCR1 sequences generated from the patient and the reference (Fig. 5e). In contrast, RT-PCR done from control RNA showed normal splicing (Fig. 5d,f). These results clearly show that the variant c.1092 + 5 A > G causes exon skipping from the pre-mRNA by interfering with the correct splicing of FLVCR1. Skipping of exon 4 is predicted to introduce a frameshift deletion of 68 base-pairs and consequently a premature termination codon in the mRNA, 31 triplets downstream of Ile343. This truncated mRNA most likely will be targeted for nonsense mediated decay.
RPGR
We detected three frameshift deletions in RPGR two of which are novel (p.Glu716Glyfs*100 and p.Gln670Argfs*24) (Table 1). The female index patient 71762 and her father were diagnosed with RP, but not her mother (Fig. 3c). The pedigree looked like a classic case of autosomal dominant RP. Sanger screening of the most common adRP associated genes RHO, PRPF31, RP1 and PRPH2 did not detect a pathogenic mutation. With WES we observed a novel heterozygous 10 base-pair deletion in RPGR (NM_001034853.1:c.2008_2017del:p.Gln670Argfs*24) that leads to a frameshift followed by truncation of the transcript, 24 triplets downstream of the mutation. Mutations in RPGR have been associated with complete expression of disease phenotype in hemizygous males and incomplete or variable expression in heterozygous carrier females14.
HARS
Mutations in this gene have recently been associated with Usher syndrome15,16. Index patient 27419 was diagnosed with Usher syndrome with no family history for the disease. We identified compound heterozygous missense variants in HARS: (i) NM_002109.5:c.262G>A:p.Gly88Ser (Novel) (ii) NM_002109.5:c.410G>A:p.Arg137Gln. This variant has been previously associated with peripheral neuropathy (HGMD accession: CM130192). Both variants substitute highly conserved amino acid residues (from Baker’s yeast to human) and are predicted to be disease-causing by three prediction programs (SIFT, MutationTaster2, Polyphen2). The variants cosegregated in the family (Fig. 4d).
Other genes
We found three novel variants in CRB1 (p.Arg744*, p.Cys183Arg, p.Cys896Ser) in two LCA patients. We also detected recurrent mutations in BEST1: homozygous p.Ala135Val and heterozygous p.Ala183Val. Mutations in BEST1 are known to be inherited in a recessive or dominant fashion. Two novel variants in USH2A (p.Pro5007Leu and p.Trp2841*) were also identified in a patient diagnosed with RP. We also observed novel and recurrent variants in PDE6B, RDH12, EYS, PROM1, ELOVL4 and RP1L1 (Table 1).
Cases where disease-causing variants were not identified: In 36% of our cases, we were not able to identify the underlying genetic cause of the disease.
Discussion
We contributed to a recent report describing a panel-based NGS approach of retinal genes17. Targeted sequencing of known genes has been successfully employed to identify disease-causing mutations in inherited RDs with variable rates of success between 60% for non-syndromic cases (e.g, RP) and 80% in syndromic cases17. Therefore, although very efficient, panel-based sequencing has its limitations. In cases where no disease-associated variants are identified within genes that have been included in the panel, other approaches have to be used to identify the genetic basis of the disease, which adds to costs and time. With 20–40% cases lacking a genetic diagnosis, the costs are significantly higher for a gene diagnostics laboratory and this may in turn lead to significantly higher costs for patients, when genetic testing is not covered by health insurance. In addition, with new genes being rapidly associated to various forms of RDs, modifying the panel becomes expensive. Therefore, we performed WES in our patients, which has the potential of identification of novel disease-associated genes. To our knowledge, this is the first report with WES data generated from human RD cases using NextSeq500. Variant distribution was comparable between both HiSeq2000 and NextSeq500 platforms; however we obtained slightly higher numbers of variants with NextSeq500. A possible reason could be the difference in the chemistries of the two platforms and the different bioinformatic algorithms used to analyze the generated data.
In this study we performed WES in 58 cases diagnosed with various RDs. Most of the patients were of Swiss origin. Wherever possible, samples from additional family members were included to perform segregation analysis. We used a stringent filtering approach that included assessment of multiple parameters such as frequency of the variants, their effect on the encoded protein, and thorough literature search for previously described variants. We identified the underlying genetic basis for the various diseases in 64% of the cases, which is comparable to previously reported panel-based approaches17,18 and sometimes even higher19. In addition to 26 recurrent mutations that have been previously associated with RDs, we detected 20 novel variants that expand the spectrum of mutations causing RDs. Amongst the 20 novel variants identified, 11 are missense variations, while others are loss of function nonsense or frameshift alterations including one variant affecting splicing. Although the bioinformatic algorithms for prediction of missense variants have their limitation, they are accepted as a first attempt to interpret pathogenic relevance of amino acid substitutions. Finally, only functional assays for the respective proteins can provide conclusive evidence. We also present novel phenotypes for known disease-associated genes, thus expanding the clinical spectrum of mutations in these genes, which is of relevance for future gene diagnostics in RD patients.
Within our patient cohort, the majority of variants were identified in ABCA4, followed by C2orf71 and RP1. Mutations in ABCA4 are predominantly known to cause STGD (currently 610 mutations in ABCA4 have been associated with STGD, source: HGMD), however mutations in ABCA4 are also associated with RP and CORD1,8,9. Although mutations in EYS seem to account for the majority of autosomal recessive RP cases (source: HGMD), in our cohort we identified EYS variants in only one case. Similarly, compared to mutations in known disease-associated genes in RD (data not shown; source: HGMD), variants in USH2A, CRB1 and PDE6B were under-represented in our dataset, while mutations in C2orf71, FLVCR1 and CEP290 were more frequent in Swiss RD patients. This difference in the frequencies of variants known to be RD-associated could be population-specific, also keeping into account the migration background within the Swiss population.
Since its first association with RP in 2010 by Collin et al.10, 29 mutations have been identified in C2orf71 that lead to arRP (source: HGMD). We identified a novel 20 base-pair frameshift deletion in the C2orf71 gene (NM_001029883.2:c.1709_1728del:p.Gly570Glufs*3) in three of our patients, either in compound heterozygous (71688, 71703) or homozygous state (29870) (Fig. 4a, Table 1). We have identified this mutation in patient 71703 and his affected brother, both of whom were diagnosed with CORD. This presents a novel disease phenotype associated with mutations in C2orf71. There is one report which associates mutations in C2orf71 with CORD type of arRP20, but not a true CORD phenotype.
A previous study, to which we contributed, had identified a variant in FLVCR1 (NM_014053.2:c.1092+5G>A) in a RP patient17. Since this variant lies within the highly conserved splice donor consensus sequence at position +5 from the 3’-end of exon 4 in FLVCR1, a defect in splicing is very likely. We performed RT-PCR experiments to evaluate the effect of the variant c.1092 + 5 G > A on the splicing of the FLVCR1 messenger RNA and found that the variant leads to skipping of exon 4 (Fig. 5). In addition, mutations in the FLVCR1 gene have been associated with posterior column ataxia with retinitis pigmentosa (PCARP). In both our patients described above (71315 and 29303), no posterior column ataxia phenotype was reported until the last clinical examination. FLVCR1 associated posterior column ataxia has been reported to manifest in the second decade of life21. Although the first patient (71315) is rather young (9 years of age), the second patient 29303 (36 years of age) has not shown any signs of neurologic disorders (until the last clinical examination done at the age of 34). It is difficult to comment on whether this might be a novel phenotype associated with mutations in FLVCR1. Alternatively, some copies of correctly spliced mRNA could be retained despite the homozygous splice-site variant in patient 29303, which might be sufficient to prevent extraocular neurological symptoms, but only leads to a retinal phenotype. Pathological significance of this exon 4 skipping is currently being studied in our laboratory in more detail. To delineate whether the two patients could be related, we compared informative SNPs which are close to the causative variant in both cases (c.1092 + 5 G > A of FLVCR1 gene). Since virtually all of the flanking SNPs were heterozygous, even in case 29303 (in whom the FLVCR1 variant c.1092 + 5 G > A is homozygous), two independent mutation events at the two different alleles are considered more likely. Otherwise, if the mutation would be derived from the same ancestral allele or haplotype, we also should observe homozygosity for SNPs flanking this variant.
In the family of case 71762, an apparent adRP revealed an X-linked inheritance pattern upon WES. The female index patient inherited from her father a pathogenic frameshift deletion mutation in RPGR on one allele. X-inactivation of the normal allele could be the most likely possibility for expression of the disease phenotype, as has been reported in earlier studies in case of RPGR14,22.
Using linkage analysis and exome sequencing, Puffenberger et al. reported a homozygous mutation in the HARS gene in patients of Swiss origin affected with Usher syndrome15. Mutations in HARS have also been associated with peripheral neuropathies. In case 27419 diagnosed with Usher syndrome, we identified compound heterozygous missense variants in HARS. Variant c.262 G > A:p.Gly88Ser was novel while the second mutation c.410 G > A:p.Arg137Gln has been shown to cause loss of function using yeast complementation assays and was associated with peripheral neuropathy23. However, this patient has not shown any signs of neurological disorders until the last clinical examination. Upon an initial screening with fewer genes of interest, we had not identified disease associated variants in this case. Only upon the association of the HARS gene with Usher syndrome, we searched for variants in Usher syndrome cases in our cohort and identified compound heterozygous pathogenic variants in HARS gene in this case. This case highlights the advantage of using WES in comparison with panel-based NGS.
Cases 30421 and 13730 highlight the limitations of WES. In both cases we identified heterozygous pathogenic mutations in CEP290 on one allele only. The second mutant allele could not be identified by exome capturing methods as it was located in a deep intronic region (c.2991 + 1655 A > G)11,12. This represents a strong case for complementing exome sequencing with other methods such as whole genome sequencing or Sanger sequencing for specific mutations.
Detection of multiple disease-associated variants in single patients
Most of our patients carried additional variants in known RD genes that either did not fit the inheritance pattern (e.g. only one recessive variant in an RD-associated gene), or did not pass one of the filtering criteria. The effect of such additional variants is difficult to predict, but these could act as disease modifiers, leading to an earlier or delayed onset/progression of the disease. A list of these variants has been added in supplementary Table 1. These variants were chosen to be pathogenic based on the same criteria that were used to identify disease associated variants.
In conclusion, we have established a highly efficient and reliable whole-exome sequencing and data analysis pipeline that delivers a high diagnostic yield of approximately 65%. In addition, WES provides the advantage that datasets can be reanalyzed as soon as a novel gene for RDs is reported. Wherever possible, we have included additional family members to confirm the diagnosis, an approach which is essential given the fact that patients frequently carry more than one pathogenic mutation in their genome. We have also detected a significant number of novel variants that will aid in further characterizing disease pathogenicity, in addition to identifying novel phenotypes for genes previously associated to some form of RD.
Materials and Methods
Patients and families
Patients and family members were referred to us from the eye clinics at the University Hospital Zürich and University Hospital Bern for genetic testing purposes. All patients or family members as well as parents of affected children provided informed consent for genetic testing according to Swiss laws. The clinical diagnosis was established by a comprehensive ophthalmological examination, functional electroretinography (ERG) and morphological studies (optical coherence tomography, OCT; fluorescein angiography, FAG; autofluorescence imaging, AF).
The study was conducted in accordance with the Helsinki Declaration. The approval for genetic testing was awarded to The Institute of Medical Molecular Genetics by the Federal Office of Public Health (FOPH) in Switzerland.
DNA extraction
Genomic DNA was isolated from patients’ blood samples according to the manufacturer’s recommendations using a coated magnetic bead technology (PerkinElmer Chemagen Technologie GmbH, Baesweiler, Germany). DNA quantification was performed using the Nanodrop (Life technologies, Darmstadt, Germany).
Whole exome sequencing (WES)
WES was performed either at Atlas Biolabs (Berlin, Germany) using Illumina HiSeq2000 or in-house using Illumina NextSeq500. Sequencing with HiSeq2000 and bioinformatics analysis was done as previously described24. For sequencing at the NextSeq500 platform, libraries were generated using Nextera Rapid Capture Exome (Illumina) to generate 150 bp paired-end reads. Alignment of sequence reads, indexing of the reference genome, variant calling and annotation was done with a pipeline based on BWA using BaseSpace Onsite (Illumina). Variants were annotated using Alamut-HT and visualized on Alamut Viewer 2.2 (Interactive Biosoftware, Rouen, France). A filtering pipeline was established to identify the most likely disease-causing variant. Since RDs are rare in the population, only variants with frequency below 1% were chosen. Frequencies of identified variants were also cross-verified on Exome Sequencing Project (ESP) data and Exome Aggregation Consortium (ExAC) data. Mutations that have been previously described to be disease-causing in Human Gene Mutation Database (HGMD) and literature were given the highest priority. The effect of mutations that were described in HGMD were further validated by reading published literature reporting the variants and only those variants that had convincing evidence were chosen. In addition, protein truncation mutations such as nonsense and frameshift (insertions or deletions), were also ranked higher in priority. For variants leading to amino acid substitution, pathogenicity predictions from five programs namely SIFT25, MAPP26, AGVGD27,28, MutationTaster229 and Polyphen230 were compared.
To make sure that medically and diagnostically important variants are not being missed, the following approach was used for cases where no disease-associated variants were identified:
-
i
Genes that have been most frequently associated with a certain disease subtype or inheritance pattern were manually screened on Alamut-viewer to check for coverage and variants that could have been missed by the variant caller. For example in patients with Leber congenital amaurosis (LCA) or Usher syndrome, exons of all genes described in Retnet to be associated with LCA or Usher were carefully screened manually.
-
ii
If an exon was identified that was not covered and in which known variants have been identified and are included in HGMD, we Sanger sequenced these uncovered exons.
-
iii
For recessive cases, if we identified a pathogenic heterozygous variant in one or more of the 252 genes, we screened all exons of these genes. We Sanger sequenced any poorly covered (<10× coverage) or uncovered exons of these genes in order to make sure that a second variant is not being missed.
RPGR OFR15
RPGR ORF15 is often poorly covered by NGS capture methods due to its repetitive nature. Patients with RPGR mutations show a severe early onset RP phenotype. All male patients diagnosed with an early onset (age <20 years) and severe RP phenotype were screened for RPGR ORF15 by conventional Sanger sequencing in our laboratory31.
Primer design, PCR amplification and Sanger sequencing
Primer design, PCR and Sanger sequencing was performed as previously described24. Sanger sequencing data analysis was performed using the Sequencing Analysis Software v5.4, Sequence Pilot (JSI Medical Systems, Germany) and SeqScape v2.6 (Applied Biosystems, Carlsbad, California, USA) to identify the likely disease causing variants. Mutation is defined as previously described17.
Additional Information
How to cite this article: Tiwari, A. et al. Next generation sequencing based identification of disease-associated mutations in Swiss patients with retinal dystrophies. Sci. Rep. 6, 28755; doi: 10.1038/srep28755 (2016).
References
Berger, W., Kloeckener-Gruissem, B. & Neidhardt, J. The molecular basis of human retinal and vitreoretinal diseases. Progress in retinal and eye research 29, 335–375, doi: 10.1016/j.preteyeres.2010.03.004 (2010).
Hartong, D. T., Berson, E. L. & Dryja, T. P. Retinitis pigmentosa. Lancet 368, 1795–1809, doi: 10.1016/S0140-6736(06)69740-7 (2006).
Venturini, G., Rose, A. M., Shah, A. Z., Bhattacharya, S. S. & Rivolta, C. CNOT3 is a modifier of PRPF31 mutations in retinitis pigmentosa with incomplete penetrance. PLoS genetics 8, e1003040, doi: 10.1371/journal.pgen.1003040 (2012).
Maubaret, C. G. et al. Autosomal dominant retinitis pigmentosa with intrafamilial variability and incomplete penetrance in two families carrying mutations in PRPF8. Investigative ophthalmology & visual science 52, 9304–9309, doi: 10.1167/iovs.11-8372 (2011).
Vithana, E. N. et al. Expression of PRPF31 mRNA in patients with autosomal dominant retinitis pigmentosa: a molecular clue for incomplete penetrance? Investigative ophthalmology & visual science 44, 4204–4209 (2003).
Dietrich, K. et al. A novel mutation of the RP1 gene (Lys778ter) associated with autosomal dominant retinitis pigmentosa. The British journal of ophthalmology 86, 328–332 (2002).
Boon, C. J. et al. The spectrum of ocular phenotypes caused by mutations in the BEST1 gene. Progress in retinal and eye research 28, 187–205, doi: 10.1016/j.preteyeres.2009.04.002 (2009).
Cideciyan, A. V. et al. Mutations in ABCA4 result in accumulation of lipofuscin before slowing of the retinoid cycle: a reappraisal of the human disease sequence. Human molecular genetics 13, 525–534, doi: 10.1093/hmg/ddh048 (2004).
Kitiratschky, V. B. et al. ABCA4 gene analysis in patients with autosomal recessive cone and cone rod dystrophies. European journal of human genetics: EJHG 16, 812–819, doi: 10.1038/ejhg.2008.23 (2008).
Collin, R. W. et al. Mutations in C2ORF71 cause autosomal-recessive retinitis pigmentosa. American journal of human genetics 86, 783–788, doi: 10.1016/j.ajhg.2010.03.016 (2010).
den Hollander, A. I. et al. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. American journal of human genetics 79, 556–561, doi: 10.1086/507318 (2006).
Perrault, I. et al. Spectrum of NPHP6/CEP290 mutations in Leber congenital amaurosis and delineation of the associated phenotype. Human mutation 28, 416, doi: 10.1002/humu.9485 (2007).
Mount, S. M. Genomic sequence, splicing, and gene annotation. American journal of human genetics 67, 788–792, doi: 10.1086/303098 (2000).
Churchill, J. D. et al. Mutations in the X-linked retinitis pigmentosa genes RPGR and RP2 found in 8.5% of families with a provisional diagnosis of autosomal dominant retinitis pigmentosa. Investigative ophthalmology & visual science 54, 1411–1416, doi: 10.1167/iovs.12-11541 (2013).
Puffenberger, E. G. et al. Genetic mapping and exome sequencing identify variants associated with five novel diseases. PloS one 7, e28936, doi: 10.1371/journal.pone.0028936 (2012).
Aparisi, M. J. et al. Targeted next generation sequencing for molecular diagnosis of Usher syndrome. Orphanet journal of rare diseases 9, 168, doi: 10.1186/s13023-014-0168-7 (2014).
Glockle, N. et al. Panel-based next generation sequencing as a reliable and efficient technique to detect mutations in unselected patients with retinal dystrophies. European journal of human genetics: EJHG 22, 99–104, doi: 10.1038/ejhg.2013.72 (2014).
Zhao, L. et al. Next-generation sequencing-based molecular diagnosis of 82 retinitis pigmentosa probands from Northern Ireland. Human genetics 134, 217–230, doi: 10.1007/s00439-014-1512-7 (2015).
Consugar, M. B. et al. Panel-based genetic diagnostic testing for inherited eye diseases is highly accurate and reproducible, and more sensitive for variant detection, than exome sequencing. Genetics in medicine: official journal of the American College of Medical Genetics 17, 253–261, doi: 10.1038/gim.2014.172 (2015).
Hebrard, M. et al. Combining gene mapping and phenotype assessment for fast mutation finding in non-consanguineous autosomal recessive retinitis pigmentosa families. European journal of human genetics: EJHG 19, 1256–1263, doi: 10.1038/ejhg.2011.133 (2011).
Rajadhyaksha, A. M. et al. Mutations in FLVCR1 cause posterior column ataxia and retinitis pigmentosa. American journal of human genetics 87, 643–654, doi: 10.1016/j.ajhg.2010.10.013 (2010).
Banin, E. et al. A non-ancestral RPGR missense mutation in families with either recessive or semi-dominant X-linked retinitis pigmentosa. American journal of medical genetics. Part A 143A, 1150–1158, doi: 10.1002/ajmg.a.31642 (2007).
Vester, A. et al. A loss-of-function variant in the human histidyl-tRNA synthetase (HARS) gene is neurotoxic in vivo . Human mutation 34, 191–199, doi: 10.1002/humu.22210 (2013).
Haghighi, A. et al. Homozygosity mapping and whole exome sequencing reveal a novel homozygous COL18A1 mutation causing Knobloch syndrome. PloS one 9, e112747, doi: 10.1371/journal.pone.0112747 (2014).
Kumar, P., Henikoff, S. & Ng, P. C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nature protocols 4, 1073–1081, doi: 10.1038/nprot.2009.86 (2009).
Stone, E. A. & Sidow, A. Physicochemical constraint violation by missense substitutions mediates impairment of protein function and disease severity. Genome research 15, 978–986, doi: 10.1101/gr.3804205 (2005).
Mathe, E. et al. Computational approaches for predicting the biological effect of p53 missense mutations: a comparison of three sequence analysis based methods. Nucleic acids research 34, 1317–1325, doi: 10.1093/nar/gkj518 (2006).
Tavtigian, S. V. et al. Comprehensive statistical study of 452 BRCA1 missense substitutions with classification of eight recurrent substitutions as neutral. Journal of medical genetics 43, 295–305, doi: 10.1136/jmg.2005.033878 (2006).
Schwarz, J. M., Cooper, D. N., Schuelke, M. & Seelow, D. MutationTaster2: mutation prediction for the deep-sequencing age. Nature methods 11, 361–362, doi: 10.1038/nmeth.2890 (2014).
Adzhubei, I. A. et al. A method and server for predicting damaging missense mutations. Nature methods 7, 248–249, doi: 10.1038/nmeth0410-248 (2010).
Neidhardt, J. et al. Identification of novel mutations in X-linked retinitis pigmentosa families and implications for diagnostic testing. Molecular vision 14, 1081–1093 (2008).
Acknowledgements
We thank all patients and their family members for participating. We also would like to thank Moreno Menghini, Silvia Azzarello-Burri, Dunja Niedrist, and Deborah Bartholdi for referrals. We are grateful to Istvan Magyar and Samuel Koller for support with in-house next generation sequencing and to Barbara Kloeckener-Gruissem for scientific discussions and suggestions. This study was funded by a grant from the Swiss National Science Foundation (Grant number: 320030_138507) to WB and JN and from Velux Stiftung Switzerland to WB.
Author information
Authors and Affiliations
Contributions
A.T., J.N. and W.B. conceived and designed the experiments. A.T. performed NGS. A.T. and Luzy Bähr performed Sanger confirmations. A.T. and A.B. analyzed sequencing data. J.F., M.S.Z., N.W., D.B., L.B. and C.G.K. recruited patients, performed clinical examination of patients and clinical interpretation. W.B. provided all reagents, materials and analysis tools. A.T. and W.B. wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Tiwari, A., Bahr, A., Bähr, L. et al. Next generation sequencing based identification of disease-associated mutations in Swiss patients with retinal dystrophies. Sci Rep 6, 28755 (2016). https://doi.org/10.1038/srep28755
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep28755
This article is cited by
-
Unearthing FLVCR1a: tracing the path to a vital cellular transporter
Cellular and Molecular Life Sciences (2024)
-
Whole genome sequencing for inherited retinal diseases in the Korean National Project of Bio Big Data
Graefe's Archive for Clinical and Experimental Ophthalmology (2023)
-
Cost-effectiveness of voretigene neparvovec in the treatment of patients with inherited retinal disease with RPE65 mutation in Switzerland
BMC Health Services Research (2022)
-
Clinical and genetic spectrums of 413 North African families with inherited retinal dystrophies and optic neuropathies
Orphanet Journal of Rare Diseases (2022)
-
Ophthalmic genetic counselling: emerging trends in practice perspectives in Asia
Journal of Community Genetics (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.