Abstract
Osteoclastogenesis is an essential process during bone metabolism which can also be promoted by inflammatory signals. Thrombomodulin (TM), a transmembrane glycoprotein, exerts anti-inflammatory activities such as neutralization of proinflammatory high-mobility group box 1 (HMGB1) through TM lectin-like domain. This study aimed to identify the role of myeloid TM (i.e., endogenous TM expression on the myeloid lineage) in osteoclastogenesis and inflammatory bone loss. Using human peripheral blood mononuclear cells and mouse bone marrow-derived macrophages, we observed that the protein levels of TM were dramatically reduced as these cells differentiated into osteoclasts. In addition, osteoclastogenesis and extracellular HMGB1 accumulation were enhanced in primary cultured monocytes from myeloid-specific TM-deficient mice (LysMcre/TMflox/flox) and from TM lectin-like domain deleted mice (TMLeD/LeD) compared with their respective controls. Micro-computerized tomography scans showed that ovariectomy-induced bone loss was more pronounced in TMLeD/LeD mice compared with controls. Finally, the inhibiting effects of recombinant TM lectin-like domain (rTMD1) on bone resorption in vitro and bone loss in both the ovariectomized model and collagen antibody-induced arthritis model has been detected. These findings suggested that the myeloid TM lectin-like domain may inhibit osteoclastogenesis by reducing HMGB1 signaling and rTMD1 may hold therapeutic potential for inflammatory bone loss.
Similar content being viewed by others
Introduction
Osteoclastogenesis is a critical process for bone resorption during bone metabolism. Precursor monocytes/macrophages can differentiate into multinuclear osteoclasts during osteoclastogenesis. Promotion of osteoclastogenesis requires two essential hematopoietic factors, i.e. macrophage colony stimulating factor (M-CSF) and receptor activator of nuclear factor kappa-B ligand (RANKL)1,2. Following activation of receptor activator of nuclear factor kappa-B (RANK) on the surface of precursor monocytes/macrophages, expression of several genes (e.g., tartrate-resistant acid phosphatase (TRAP), cathepsin K (CATK), calcitonin receptor and β3integrin) can be induced in the osteoclasts2,3. Other cells such as activated T-cells can modulate the formation of osteoclasts by secreting RANKL or osteoprotegerin, suggesting that the immune system might affect bone metabolism and homeostasis. This interaction has led to an interdisciplinary research field known as osteoimmunology that investigates the crosstalk between immune/inflammatory responses and bone metabolism4.
Thrombomodulin (TM) was first discovered as a membrane glycoprotein that activates protein C as an anti-coagulant5. TM belongs to the C-type lectin-like domain superfamily and is expressed in various cell types such as keratinocytes, endothelial cells, myeloid-derived monocytes and macrophages6,7,8. Previous studies have demonstrated that TM participates in various biological processes such as cell-cell adhesion, epithelial-mesenchymal transition, keratinocyte differentiation and cutaneous wound healing and inflammation9,10,11,12. From the N- to C-terminus, TM is composed of five functional domains, including a C-type lectin-like domain (domain 1, TMD1), a domain with six epidermal growth factor (EGF)-like structures (domain 2, TMD2), a serine/threonine-rich domain (domain 3, TMD3), a transmembrane domain (domain 4, TMD4) and a cytoplasmic domain (domain 5, TMD5)13. The soluble forms of TM (sTM) have been considered a marker of organ dysfunction and a novel angiogenic factor14,15. The expression of TM is tightly regulated by inflammatory responses16. TM also exerts anti-inflammatory activities where TMD1 may sequester the pro-inflammatory high-mobility group box 1 (HMGB1) protein. This would prevent HMGB1 from engaging its receptors that may result in sustained chronic inflammatory responses and eventually in tissue damage17. In addition, TM aids the proteolytic cleavage of HMGB1 by thrombin18. Our previous study also demonstrated that recombinant TMD1 (rTMD1) suppresses inflammation by directly binding to lipopolysaccharide (LPS)19. Taken together, these studies reveal that TM functional domains can modulate inflammatory responses. Recently, endosialin (CD248), another member of the C-type lectin-like domain containing proteins, has been reported as a negative regulator of bone formation in mice20,21. However, the significance of TM during osteoclastogenesis is not clear.
Macrophages are derived from monocytes that represent circulating cell members of the myeloid lineage. In this study, we examined the hypothesis that the myeloid TM lectin-like domain negatively regulates osteoclastogenesis. RAW264.7 cells, a mouse macrophage cell line and isolated human peripheral blood mononuclear cells (PBMCs) were used to identify the expression pattern of TM during osteoclastogenesis in vitro. In addition, the functions of TM and its lectin-like domain during the formation of osteoclasts were investigated using primary cultured monocytes/macrophages from the TM transgenic mice LysMcre/TMflox/flox (myeloid-specific TM-deficient mice) and TMLeD/LeD (TM lectin-like domain deleted mice), along with their respective controls. Furthermore, the potential mechanism by which TM suppresses osteoclastogenesis was explored. Finally, the therapeutic effects of rTMD1 on bone resorption in vitro and bone loss in vivo were evaluated. The results would elucidate the significance of myeloid TM and its lectin-like domain in osteoporosis.
Results
TM protein expression in monocytes/macrophages was reduced during osteoclastogenesis
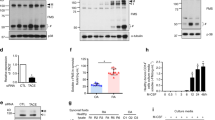
Osteoclastogenesis in mouse RAW 264.7 cells and human PBMCs was induced using RANKL and M-CSF to evaluate the levels of TM protein during the process. Immunofluorescence staining revealed that TM expression was dramatically decreased as RAW 264.7 cells differentiated into osteoclast-like cells (Fig. 1A). Western blot analysis showed that treatment of RAW 264.7 cells with RANKL reduced TM but increased CATK, a marker of osteoclasts, in a time-dependent manner (Fig. 1B,C). Moreover, similar results were observed in human PBMCs (Fig. 1D–F). These results suggested that TM expression in monocytes/macrophages may be inversely related to osteoclastogenesis.
Down-regulated TM expression in mammalian osteoclast-like cells.
Immunofluorescence staining of TM expression in RAW 264.7 cells, PBMCs and their differentiated osteoclast-like cells induced by treatment with M-CSF (20 ng/mL) and RANKL (30 ng/mL) for (A) 4 days and for (D) 1 week (original magnification × 200). Western blot analysis of TM and CATK expression in (B) RAW 264.7 cells and (E) PBMCs at indicated times after treatment with M-CSF and RANKL. Representative figures from three independent experiments are shown. (C,F) Quantitative representation of (B,E). Statistics were performed by Student’s t-test. **P < 0.01, ***P < 0.001.
Deficiency of full-length TM in macrophages enhanced the RANKL-induced osteoclastogenesis
To investigate whether TM might be a negative regulator in osteoclastogenesis, macrophages from myeloid-specific TM-deficient mice were isolated. TRAP staining showed that RANKL-induced osteoclastogenesis in LysMcre/TMflox/flox macrophages was more effective than that from TMflox/flox macrophages (Fig. 2A). Quantification of the results showed that the ratio of differentiated TRAP positive multinucleated cells (TRAP+ MNCs) in TM-deficient macrophages was at least 3-fold higher compared with those in TM-wildtype macrophages (Fig. 2B). Moreover, TRAP activities in TM-deficient macrophages were significantly higher than those in TM-wildtype macrophages (Fig. 2C). These results indicated that the expression of full-length TM in macrophages may hinder RANKL-induced osteoclastogenesis.
Myeloid-specific TM deletion enhanced osteoclasts formation.
(A) TRAP staining of osteoclasts differentiated from primary cultured TMflox/flox and LysMcre/TMflox/flox mice macrophages, treated with M-CSF and various concentrations of RANKL for differentiation (original magnification × 200). (B) Calculated ratio of TRAP+ MNCs to total cells per well. (C) Quantitation of TRAP activity using 4-NPP as substrate. Statistics were performed by Two-way ANOVA. *P < 0.05, ***P < 0.001. Data showed the mean ± SD for three independent experiments.
RANKL-induced osteoclastogenesis inhibited by myeloid TM lectin-like domain
It has been showed that osteoblast-derived C-type lectin could inhibit osteoclast formation22. To further investigative whether the lectin-like domain of myeloid TM contributed to RANKL-induced osteoclastogenesis inhibition, primary macrophages from TM lectin-like domain-deficient TMLeD/LeD mice and their controls TMWT/LeD mice were obtained. TRAP staining showed that RANKL-induced osteoclastogenesis was more prominent in macrophages from TMLeD/LeD mice than those in TMWT/LeD mice (Fig. 3A). Also, the TRAP+ MNCs ratio and TRAP activities in TM lectin-like domain-deficient macrophages were significantly higher than those in controls (Fig. 3B,C). These results suggested that the lectin-like domain of myeloid TM may have a critical effect on the inhibition of RANKL-induced osteoclastogenesis.
Inhibition of RANKL-induced osteoclast formation by TM lectin-like domain on macrophages.
(A) TRAP staining of osteoclasts differentiated from primary cultured TMWT/LeD and TMLeD/LeD mice macrophages, treated with M-CSF and various concentrations of RANKL for differentiation (original magnification × 200). (B) Ratio of TRAP+ MNCs to total cells per well. (C) Quantitation of TRAP activity using 4-NPP as substrate. Statistics were performed by Two-way ANOVA. **P < 0.01, ***P < 0.001. Experiments were repeated three times.
Deletion of the full-length TM or TM lectin-like domain enhanced HMGB1 secretion and bone resorption in osteoclasts and increased OVX-induced serum HMGB1 level and bone loss
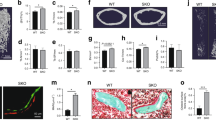
Since HMGB1 release may be critical for osteoclastogenesis and rheumatic diseases23,24, we further evaluated the effects of TM deficiency on HMGB1 translocation, secretion and bone loss. In TMflox/flox cells, RANKL treatment enhanced cytoplasmic translocation of HMGB1 in the presence of M-CSF (Fig. 4A). The cytoplasmic fraction and total expression level of HMGB1 were increased in LysMcre/TMflox/flox cells compared with TMflox/flox cells while treated with M-CSF only. Similar results were observed when LysMcre/TMflox/flox cells and TMflox/flox were treated with M-CSF and RANKL, suggesting that full-length TM deletion may contribute to HMGB1 translocation and production in osteoclasts. In addition, RANKL-enhanced HMGB1 production in LysMcre/TMflox/flox and TMLeD/LeD cells increased more than 3-fold compared with their respective controls (Fig. 4B,C). Bone resorption activities, as indicated by pit area and fluorescence intensity, were significantly enhanced in isolated TMLeD/LeD cells compared with TMWT/LeD cells (Fig. 4D,E). Similarly, serum HMGB1 level in TMLeD/LeD was significantly higher than that in TMWT/LeD mice (Fig. 4F). In addition, μCT scanning showed that OVX-induced tibia bone loss was significantly more severe in TMLeD/LeD mice than in TMWT/LeD mice (Fig. 4G,H). The expression levels of CATK and the number of osteoclasts were elevated in the bone section of TMLeD/LeD mice as compared with TMWT/LeD mice (Fig. 4I,J). These data suggested that deficiency of myeloid TM lectin-like domain led to enhanced bone resorption and bone loss, probably in association with extracellular HMGB1 production.
TM deficiency enhanced HMGB1 translocation and secretion, bone resorption and ovariectomy-induced bone loss.
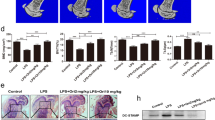
(A) After 24-hour treatment, whole-cell lysates were fractionated into nuclear (N) and cytosolic (C) fractions. The localization of HMGB1 was analyzed by western blotting. Evaluation of HMGB1 secretion by western blot analysis in primary cultured macrophages from (B) TMflox/flox and LysMcre/TMflox/flox mice and from (C) TMWT/LeD and TMLeD/LeD mice. GST was added as internal control. CM, conditioned medium. Results of bone resorption assay were obtained by measuring the (D) pit area in fluoresceinamine-labeled chondroitin sulfate/calcium phosphate-coated plates and (E) fluorescence intensity in conditioned medium. (F) Detected HMGB1 levels in serum by ELISA assay after 4 weeks of ovariectomy. (G) Bone loss in tibia was detected by μCT scanning after 4 weeks of ovariectomy. Dashed lines indicated the cross sections. (H) Quantitative results of trabecular bone volume (BV), total bone volume (TV) and BV/TV in (G). n = 5 each group. (I) Immunohistochemical staining for cathepsin K in the tibia (brown). bar = 100 μm. (J) Osteoclast number/bone surface (N.Oc/BS, N/mm) were measured. Statistics were performed by Student’s t-test. *P < 0.05, **P < 0.01.
Treatment with rTMD1 inhibited bone resorption and OVX-induced bone loss
TMD1 has been demonstrated to neutralize HMGB1-triggered inflammation17. Therefore, we investigated the effects of human rTMD1 on bone resorption in vitro and bone loss in vivo. Treatment with rTMD1 in RAW264.7 cells significantly inhibited RANKL-induced TRAP activity (Fig. 5A) and dose-dependently reduced bone resorption activity (Fig. 5B). Furthermore, rTMD1 treatment also attenuated extracellular HMGB1 production in TMLeD/LeD cells (Fig. 5C). In vivo, rTMD1 treatment suppressed bone loss across a 4-week period and increased the bone volume fraction (BV/TV, %) in a dose-dependent manner (Fig. 5D–F). Furthermore, histomorphometric analysis showed that the bone loss was reduced by treatment with rTMD1 (Fig. 5G). These results suggested that rTMD1 inhibited bone loss, at least in part, through blockade of HMGB1-induced osteoclastogenesis.
Recombinant TM lectin-like domain (rTMD1) suppressed RANKL-induced bone resorption and reduced ovariectomy-induced bone loss.
(A) RANKL-induced TRAP activity in RAW264.7 cells was measured after rTMD1 treatment for 4 days. (B) Results of RANKL-induced bone resorption in RAW264.7 cells after treatment with various doses of rTMD1. (C) Western blotting analysis of extracellular HMGB1 from TMLeD/LeD mice BMMs in response to rTMD1 treatment. CM, conditioned medium; Lys, cell lysates. (D) After OVX, an intraperitoneal injection of rTMD1 (1 mg/kg) was performed every 3 days until sacrificed. Bone loss in tibia was detected by μCT scanning in wild-type mice with or without rTMD1 treatment, within 4 weeks of ovariectomy. (E) Quantified BV/TV of (D). n = 5. (F) Quantitative results of BV/TV in ovariectomized wild-type mice treated with various doses of intraperitoneal rTMD1 injection (0.5 mg/kg, 1 mg/kg, 3 mg/kg). n = 5. BV, trabecular bone volume; TV, total bone volume. (G) H&E staining of the tibia after 4 weeks of ovariectomy. bar = 50 μm. Student’s t-test (A); One-way ANOVA (B), (F); Two-way ANOVA (E). *P < 0.05, **P < 0.01, ***P < 0.001.
Treatment with rTMD1 inhibited bone loss in CAIA mice
Finally, the therapeutic effects of rTMD1 treatment in the CAIA model, a model featured with joint inflammation and generalized bone loss25, were also evaluated. The experimental design was showed in Fig. 6A. Our results showed that rTMD1 treatment significantly reduced the paw thickness (Fig. 6B,C), arthritis score (Fig. 6D) and levels of serum HMGB1 (Fig. 6E). Compared with mice treated with PBS, those supplemented with rTMD1 had significantly higher bone volume fraction (BV/TV, %) (Fig. 6F) and trabecular bone number (Tb. N, 1/mm) (Fig. 6G). Taken together, these data suggested that rTMD1 treatment may inhibit inflammatory bone loss in the CAIA model.
Treatment with rTMD1 inhibited bone loss and reduced serum HMGB1 in CAIA mouse model.
(A) Experimental design of CAIA and rTMD1 treatment (1 mg/kg). (B) The front paws of CAIA mice with and without rTMD1 treatment. (C) Paw thickness. (D) Arthritis score. (E) Results of HMGB1 serum levels in mouse with or without rTMD1 treatment. (F) Quantitative results of BV/TV. (G) Quantitative results of Tb. N. n = 3. Student’s t-test (E–G). *P < 0.05, **P < 0.01. (H) A schematic model of myeloid TM in osteoclastogenesis and inflammatory bone loss. During osteoclastogenesis, RANKL reduced TM expression and promoted extracellular HMGB1 secretion in monocytes, which resulted in the enhancement of bone resorption and bone loss. In addition, rTMD1 treatment could inhibit HMGB1 secretion, bone resorption and bone loss.
Discussion
Osteoporosis is a disorder caused by increased bone resorption and reduced bone formation26. Normal bone resorption coupled with reduced synthesis of bone matrix may be considered as low-turnover osteoporosis. In contrast, high-turnover osteoporosis indicates a condition where increased bone resorption results from increased osteoclast activity. Low-turnover osteoporosis is generally induced by corticosteroids, whereas high-turnover osteoporosis is associated with estrogen deficiency or rheumatoid arthritis and other chronic inflammatory disorders27,28.
In this study, we demonstrated that myeloid TM was reduced during osteoclastogenesis (Fig. 1), implicating a role of myeloid TM in high-turnover osteoporosis. RANKL-induced osteoclastogenesis was promoted in full-length TM-deficient macrophages. In addition, RANKL-induced osteoclastogenesis was promoted in macrophages with specific deletion of TM lectin-like domain. These observations suggested that full-length TM in macrophages may inhibit osteoclastogenesis and that this inhibitory effect likely resulted from its lectin-like domain.
RANKL is one of the key regulatory molecules in osteoclast formation through activation of intracellular NF-κB signaling in osteoclast precursor cells. NF-κB is a critical mediator of TM repression by cytokines29. Therefore, it is plausible that through the activation of NF-κB, RANKL induced TM down-regulation in monocytes/macrophages. In our study, genetic deletion of TM in monocytes/macrophages led to the promotion of osteoclastogenesis (Figs 2 and 3). Previous reports have demonstrated that long-standing diabetes results in osteoporosis in rats and diabetic patients have a high risk for osteoporotic fractures30,31. Other studies have indicated that a high-glucose environment may activate NF-κB, which may suppress TM expression32,33. These findings suggested that diseases with suppressed TM expression may increase osteoporosis risk. Along with these findings, our study may provide a new perspective in explaining diabetes-related bone loss.
Extracellular HMGB1, which can be actively released by activated monocytes/macrophages, is a critical factor for RANKL-induced osteoclastogenesis23,34. HMGB1 is also involved in a positive feedback mechanism to sustain inflammation35. In the present study, myeloid-specific TM knockout enhanced extracellular HMGB1 production in vitro. Similarly, increased HMGB1 secretion, bone resorption and bone loss were also observed in mice with whole-body TM lectin-like domain deletion (Fig. 4). These suggesting that the mechanisms by which TM regulates osteoclastogenesis and bone remodeling may involve, at least in part, the inhibition of extracellular HMGB1 production. Moreover, rTMD1 treatment reduced the extracellular HMGB1 levels and bone loss not only in the ovariectomized model, but also in the CAIA model (Figs 5 and 6). This is consistent with a previous study that showed rTMD1 suppressed inflammation and arthritis in mouse model36.
HMGB1 deficient and conditional knockout mice have been generated in previous studies. In one study, HMGB1-deficient mice may die within 24 hours after birth due to insufficient glucocorticoid receptor expression and hypoglycaemia37. In the other study, results obtained from HMGB1 conditional knockout mice showed that myeloid HMGB1 contributes to protection from endotoxemia and bacterial infection in mice38. Based on the observations in our study, further studies using HMGB1 conditional knockout mice to study the definitive role of HMGB1 in osteoclastogenesis and bone remodeling are warranted.
Estrogen replacement is the traditional therapy used to prevent bone loss and fractures39. However, the risk of breast cancer may be accentuated following estrogen therapy40. Currently, other therapeutic approaches are developed to inhibit excessive bone resorption, such as bisphosphonates or antibodies of RANKL or CATK, while intermittent administration of parathyroid hormone is developed to promote bone formation41,42. In our study, rTMD1 suppresses osteoclastogenesis and holds promise as an effective therapeutic agent for osteoporosis.
In summary, we demonstrated for the first time that myeloid TM lectin-like domain functions as a key inhibitor of HMGB1-mediated osteoclastogenesis (Fig. 6E). Therefore, administration of recombinant TM lectin-like domain may hold therapeutic potential for postmenopausal osteoporosis or inflammation-related bone loss.
Methods
Reagents
Antibodies and recombinant proteins were purchased from the following companies: anti-tubulin antibody (Calbiochem EMD Biosciences, La Jolla, California, USA); anti-TRAP antibody (Invitrogen Carlsbad, CA, USA); 4-nitrophenyl phosphate (4-NPP), anti-TM antibody, anti-cathepsin K (CATK) antibody, anti-actin antibody, anti-glutathione S-transferase (GST) antibody, anti-lamin B antibody and RANKL protein (Santa Cruz, CA, USA); ArthritoMab™ CII mAb cocktail (MD Biosciences GmbH, Zürich, Switzerland); and M-CSF protein (R&D System, Minneapolis, MN, USA). Lipopolysaccharide (LPS) was purchased from MD Biosciences. RAW264.7 cells were purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA). The TM domains were prepared in our laboratory as previously described43.
Experimental animals
LysMcre/TMflox/flox mice with myeloid-specific TM deletion were produced as previously reported9. LysMcre/TMflox/flox mice had their TM expression deletion in the myeloid lineage except for other tissues and organs, whereas TMflox/flox mice had no TM suppression. Mice lacking the lectin-like domain of TM (TMLeD/LeD mice) and their controls (TMWT/LeD mice) were a gift from Dr. Conway44. These B6-background mice were maintained in a pathogen-free animal facility at the Animal Center of National Cheng Kung University (NCKU). Collagen antibody-induced arthritis (CAIA) was performed in C57BL/6 mice, along with bilateral ovariectomy (OVX) and evaluation of the paw thickness and arthritis scores as previously reported25. OVX was performed at 8 weeks of age. Histomorphometric analysis of the tibia was performed on paraffin sections after decalcification and the number of osteoclasts/bone surface (N.Oc/BS, N/mm) was counted as previously described45. All animal protocols were approved by the Institutional Animal Care and Use Committee of NCKU. The methods were carried out in accordance with the approved guidelines.
Cell isolation and cell culture
Human PBMCs were separated from whole blood using a Ficoll-Paque PLUS (GE Healthcare, Brussels, Belgium) gradient based on the manufacturer’s instructions. Appropriate informed consent was obtained from the volunteers. The study protocols and consent documents were approved by the Institutional Review Board of NCKU Hospital. Mouse bone marrow macrophages/monocytes (BMMs) were isolated from the femur and tibia (6 to 12 weeks old) as previously described with some modifications46. Briefly, isolated total bone marrow cells were incubated overnight in complete Minimum Essential Medium (Alpha modification; α-MEM). Subsequently, nonadherent cells were collected and mononuclear cells were prepared using Ficoll-Hypaque (GE Healthcare, Piscataway, NJ, USA) density gradient centrifugation. The BMMs were evenly scattered across the interface between Ficoll-Hypaque and medium. Cells were cultured in α-MEM supplemented with 5% heat-inactivated fetal bovine serum (FBS), L-glutamine (292 mg/L) and 1% antibiotics (penicillin and streptomycin; Invitrogen, Carlsbad, CA, USA). The methods were carried out in accordance with the approved guidelines.
Osteoclast culture, conditioned medium collection, TRAP staining
Cells (RAW264.7, PBMCs, or BMMs) were cultured in 96-well plates (1 × 105 cells/well). RANKL and M-CSF were added to stimulate osteoclast generation. Media were replenished every 2 days. After 24 hours of treatment, the conditioned medium was concentrated using Centricon tubes (Amicon, Beverly, MA) and GST was added (20 μg per sample) as an internal control. The concentrated samples were then subjected to western blot analysis. On day 4 or day 7, cells were fixed with 3% formaldehyde and were stained with TRAP (Sigma, St. Louis, MO). The ratio of TRAP-positive multinucleated cells (>4 nuclei/cell) to total cells in each well was calculated47.
Subcellular fractionation
Subcellular fractionation was performed following the protocol by Abcam (http://www.abcam.com/ps/pdf/protocols/subcellular_fractionation.pdf). Briefly, cells were lysed with a subcellular fractionation buffer and cell lysates were centrifuged. After centrifuge, the pellet was resuspended in nuclear buffer and the nuclear fraction was obtained. The supernatant was re-centrifuged at a higher speed (40,000 rpm). The cytoplasmic fraction was obtained after ultra-centrifugation.
TRAP activity assay
TRAP activity was measured using 4-NPP as substrate, based on the microplate assay method with modifications48. A 50 μL cell lysate was incubated with 150 μL of substrate (8 mM 4-NPP, 100 mM sodium acetate and 500 mM sodium tartrate, pH 5.0) for 40 min at 37 °C. The reaction was terminated by the addition of 50 μL 3 M NaOH. Absorbance was measured at 405 nm in a microplate reader (SPECTRAmax™340; Molecular Devices, Palo Alto, CA).
Western blot analysis
Cells were lysed and western blot analysis was performed as previously described49. Approximately 50 μg of total protein was separated on 10% sodium dodecyl sulfate-polyacrylamide gel and transferred onto a polyvinylidene difluoride membrane (Amersham Pharmacia Biotech, Buckinghamshire, UK). After probing with a primary and a secondary antibody, the signal was detected using an enhanced chemiluminescence reagent (Amersham Pharmacia Biotech) and results were quantified using ImageJ software (National Institutes of Health, Bethesda, MD).
Bone resorption assay
Bone resorption activities were determined by the bone resorption assay kit (Cosmo Bio. Co. Ltd., Tokyo, Japan) based on the manufacturer’s instructions. In brief, cells were incubated on fluoresceinated calcium phosphate-coated microplate with M-CSF (30 ng/mL) and RANKL (30 ng/mL) in the presence or absence of various concentrations of rTMD1 for 7 days. RANKL-induced bone resorption activity was evaluated by measuring pit area of each well and by detecting the fluorescence intensity of conditioned medium at an excitation wavelength of 485 nm and an emission wavelength of 535 nm.
Quantification of serum HMGB1
Serum levels of HMGB1 were detected from 10 μL sera using HMGB1 ELISA kit II (Shino-Test Corp., Kanagawa, Japan) according to the manufacturer’s instructions.
Micro-Computed Tomography (μCT)
All μCT analyses performed in this study were consistent with the current guidelines50. Bone samples from all groups were imaged using a SkyScan-1076 Micro-CT System (Skyscan, Kontich, Belgium). For trabecular bone analysis, the μCT scanner was operated at 45 kV, 220 μA, 0.4 μ rotation step, 0.5 mm aluminum filter and a scan resolution of 18 μm/pixel. The following 3D parameters were measured by software CT Analyser (Belgium): total bone volume (TV, mm3), trabecular bone volume (BV, mm3), trabecular bone volume fraction (BV/TV, %) and trabecular bone number (Tb. N, 1/mm).
Statistical analysis
Data were expressed as mean ± standard deviation (SD). Student’s t test or analysis of variance (ANOVA) was used to assess the statistical significance for the respective data. Asterisks in the figures were used to indicate the levels of significance: *p < 0.05, **p < 0.005 and ***p < 0.001.
Additional Information
How to cite this article: Cheng, T.-L. et al. Myeloid thrombomodulin lectin-like domain inhibits osteoclastogenesis and inflammatory bone loss. Sci. Rep. 6, 28340; doi: 10.1038/srep28340 (2016).
References
Yasuda, H. et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 95, 3597–3602 (1998).
Lacey, D. L. et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93, 165–176 (1998).
Nakagawa, N. et al. RANK is the essential signaling receptor for osteoclast differentiation factor in osteoclastogenesis. Biochem Biophys Res Commun 253, 395–400, 10.1006/bbrc.1998.9788 (1998).
Arron, J. R. & Choi, Y. Bone versus immune system. Nature 408, 535–536, 10.1038/35046196 (2000).
Dittman, W. A. & Majerus, P. W. Structure and function of thrombomodulin: a natural anticoagulant. Blood 75, 329–336 (1990).
McCachren, S. S., Diggs, J., Weinberg, J. B. & Dittman, W. A. Thrombomodulin expression by human blood monocytes and by human synovial tissue lining macrophages. Blood 78, 3128–3132 (1991).
Raife, T. J. et al. Thrombomodulin expression by human keratinocytes. Induction of cofactor activity during epidermal differentiation. J Clin Invest 93, 1846–1851, 10.1172/JCI117171 (1994).
Stearns-Kurosawa, D. J., Kurosawa, S., Mollica, J. S., Ferrell, G. L. & Esmon, C. T. The endothelial cell protein C receptor augments protein C activation by the thrombin-thrombomodulin complex. Proc Natl Acad Sci USA 93, 10212–10216 (1996).
Ma, C. Y. et al. Monocytic thrombomodulin triggers LPS- and gram-negative bacteria-induced inflammatory response. J Immunol 188, 6328–6337, 10.4049/jimmunol.1102266 (2012).
Huang, H. C. et al. Thrombomodulin-mediated cell adhesion: involvement of its lectin-like domain. J Biol Chem 278, 46750–46759, 10.1074/jbc.M305216200 (2003).
Kao, Y. C. et al. Downregulation of thrombomodulin, a novel target of Snail, induces tumorigenesis through epithelial-mesenchymal transition. Mol Cell Biol 30, 4767–4785, 10.1128/MCB.01021-09 (2010).
Cheng, T. L. et al. Thrombomodulin regulates keratinocyte differentiation and promotes wound healing. J Invest Dermatol 133, 1638–1645, 10.1038/jid.2013.8 (2013).
Weiler, H. & Isermann, B. H. Thrombomodulin. J Thromb Haemost 1, 1515–1524 (2003).
Shi, C. S. et al. Evidence of human thrombomodulin domain as a novel angiogenic factor. Circulation 111, 1627–1636, 10.1161/01.CIR.0000160364.05405.B5 (2005).
Gando, S., Kameue, T., Nanzaki, S. & Nakanishi, Y. Cytokines, soluble thrombomodulin and disseminated intravascular coagulation in patients with systemic inflammatory response syndrome. Thromb Res 80, 519–526 (1995).
Conway, E. M. Thrombomodulin and its role in inflammation. Semin Immunopathol 34, 107–125, 10.1007/s00281-011-0282-8 (2012).
Abeyama, K. et al. The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J Clin Invest 115, 1267–1274, 10.1172/JCI22782 (2005).
Ito, T. et al. Proteolytic cleavage of high mobility group box 1 protein by thrombin-thrombomodulin complexes. Arterioscler Thromb Vasc Biol 28, 1825–1830, 10.1161/ATVBAHA.107.150631 (2008).
Shi, C. S. et al. Lectin-like domain of thrombomodulin binds to its specific ligand Lewis Y antigen and neutralizes lipopolysaccharide-induced inflammatory response. Blood 112, 3661–3670, 10.1182/blood-2008-03-142760 (2008).
Naylor, A. J. et al. The mesenchymal stem cell marker CD248 (endosialin) is a negative regulator of bone formation in mice. Arthritis Rheum 64, 3334–3343, 10.1002/art.34556 (2012).
Greenlee, M. C., Sullivan, S. A. & Bohlson, S. S. CD93 and related family members: their role in innate immunity. Curr Drug Targets 9, 130–138 (2008).
Zhou, H. et al. A novel osteoblast-derived C-type lectin that inhibits osteoclast formation. J Biol Chem 276, 14916–14923, 10.1074/jbc.M011554200 (2001).
Zhou, Z. et al. HMGB1 regulates RANKL-induced osteoclastogenesis in a manner dependent on RAGE. J Bone Miner Res 23, 1084–1096, 10.1359/jbmr.080234 (2008).
Harris, H. E., Andersson, U. & Pisetsky, D. S. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat Rev Rheumatol 8, 195–202, 10.1038/nrrheum.2011.222 (2012).
Grahnemo, L. et al. Trabecular bone loss in collagen antibody-induced arthritis. Arthritis Res Ther 17, 189, 10.1186/s13075-015-0703-5 (2015).
Seeman, E. Reduced bone formation and increased bone resorption: rational targets for the treatment of osteoporosis. Osteoporos Int 14 Suppl 3, S2–8, 10.1007/s00198-002-1340-9 (2003).
Raisz, L. G. Pathogenesis of osteoporosis: concepts, conflicts and prospects. J Clin Invest 115, 3318–3325, 10.1172/JCI27071 (2005).
Wu, Q. et al. Secondary osteoporosis in collagen-induced arthritis rats. J Bone Miner Metab, 10.1007/s00774-015-0700-4 (2015).
Sohn, R. H. et al. Regulation of endothelial thrombomodulin expression by inflammatory cytokines is mediated by activation of nuclear factor-kappa B. Blood 105, 3910–3917, 10.1182/blood-2004-03-0928 (2005).
Verhaeghe, J., Visser, W. J., Einhorn, T. A. & Bouillon, R. Osteoporosis and diabetes: lessons from the diabetic BB rat. Horm Res 34, 245–248 (1990).
Montagnani, A., Gonnelli, S., Alessandri, M. & Nuti, R. Osteoporosis and risk of fracture in patients with diabetes: an update. Aging Clin Exp Res 23, 84–90 (2011).
Morigi, M. et al. Leukocyte-endothelial interaction is augmented by high glucose concentrations and hyperglycemia in a NF-kB-dependent fashion. J Clin Invest 101, 1905–1915, 10.1172/JCI656 (1998).
Cheng, T. L. et al. Thrombomodulin promotes diabetic wound healing by regulating toll-like receptor 4 expression. J Invest Dermatol 135, 1668–1675, 10.1038/jid.2015.32 (2015).
Gardella, S. et al. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep 3, 995–1001, 10.1093/embo-reports/kvf198 (2002).
van Beijnum, J. R., Buurman, W. A. & Griffioen, A. W. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1). Angiogenesis 11, 91–99, 10.1007/s10456-008-9093-5 (2008).
Van de Wouwer, M. et al. The lectin-like domain of thrombomodulin interferes with complement activation and protects against arthritis. J Thromb Haemost 4, 1813–1824, 10.1111/j.1538-7836.2006.02033.x (2006).
Calogero, S. et al. The lack of chromosomal protein Hmg1 does not disrupt cell growth but causes lethal hypoglycaemia in newborn mice. Nat Genet 22, 276–280, 10.1038/10338 (1999).
Yanai, H. et al. Conditional ablation of HMGB1 in mice reveals its protective function against endotoxemia and bacterial infection. Proc Natl Acad Sci USA 110, 20699–20704, 10.1073/pnas.1320808110 (2013).
Ettinger, B., Genant, H. K. & Cann, C. E. Long-term estrogen replacement therapy prevents bone loss and fractures. Ann Intern Med 102, 319–324 (1985).
Schairer, C. et al. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. JAMA 283, 485–491 (2000).
Rachner, T. D., Khosla, S. & Hofbauer, L. C. Osteoporosis: now and the future. Lancet 377, 1276–1287, 10.1016/S0140-6736(10)62349-5 (2011).
Im, G. I., Qureshi, S. A., Kenney, J., Rubash, H. E. & Shanbhag, A. S. Osteoblast proliferation and maturation by bisphosphonates. Biomaterials 25, 4105–4115, 10.1016/j.biomaterials.2003.11.024 (2004).
Lai, C. H. et al. Recombinant human thrombomodulin suppresses experimental abdominal aortic aneurysms induced by calcium chloride in mice. Ann Surg 258, 1103–1110, 10.1097/SLA.0b013e31827df7cb (2013).
Conway, E. M. et al. The lectin-like domain of thrombomodulin confers protection from neutrophil-mediated tissue damage by suppressing adhesion molecule expression via nuclear factor kappaB and mitogen-activated protein kinase pathways. J Exp Med 196, 565–577 (2002).
Zhang, Y. et al. Amlexanox Suppresses Osteoclastogenesis and Prevents Ovariectomy-Induced Bone Loss. Sci Rep 5, 13575, 10.1038/srep13575 (2015).
Lee, S. K., Gardner, A. E., Kalinowski, J. F., Jastrzebski, S. L. & Lorenzo, J. A. RANKL-stimulated osteoclast-like cell formation in vitro is partially dependent on endogenous interleukin-1 production. Bone 38, 678–685, 10.1016/j.bone.2005.10.011 (2006).
Toyosaki-Maeda, T. et al. Differentiation of monocytes into multinucleated giant bone-resorbing cells: two-step differentiation induced by nurse-like cells and cytokines. Arthritis Res 3, 306–310 (2001).
Lau, K. H., Onishi, T., Wergedal, J. E., Singer, F. R. & Baylink, D. J. Characterization and assay of tartrate-resistant acid phosphatase activity in serum: potential use to assess bone resorption. Clin Chem 33, 458–462 (1987).
Cheng, T. L. et al. Functions of rhomboid family protease RHBDL2 and thrombomodulin in wound healing. J Invest Dermatol 131, 2486–2494, 10.1038/jid.2011.230 (2011).
Bouxsein, M. L. et al. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 25, 1468–1486, 10.1002/jbmr.141 (2010).
Acknowledgements
This work was supported, in part, by the Ministry of Science and Technology (Taipei, Taiwan; grants NSC 102-2325-B-006-002- for Hua-Lin Wu and MOST 104-2320-B-037-026 – for Tsung-Lin Cheng). This research was, in part, supported by the Ministry of Education, Taiwan, ROC., The Aim for the Top University Project to the National Cheng Kung University and by Kaohsiung Medical University “Aim for the Top Universities Grant, grant No. KMU-TP104B10 and KMU-TP104B13”. The authors thank the Center for Research Resources and Development in Kaohsiung Medical University for the assistance in μCT analysis.
Author information
Authors and Affiliations
Contributions
T.C. and C.L. designed and performed experiments, analyzed data, generated figures and wrote the manuscript. Y.J. performed statistical analyses and generated figures. S.S. collected samples. J.Y., A.Y., Y.W., C.W., C.C. and M.H. designed experiments and analyzed and interpreted data. G.S. and H.W. designed and analyzed experiments and contributed to the writing of the manuscript. All authors approved the final draft.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Cheng, TL., Lai, CH., Shieh, SJ. et al. Myeloid thrombomodulin lectin-like domain inhibits osteoclastogenesis and inflammatory bone loss. Sci Rep 6, 28340 (2016). https://doi.org/10.1038/srep28340
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep28340
This article is cited by
-
Role of non-macrophage cell-derived HMGB1 in oxaliplatin-induced peripheral neuropathy and its prevention by the thrombin/thrombomodulin system in rodents: negative impact of anticoagulants
Journal of Neuroinflammation (2019)
-
Role of Thrombin in Soluble Thrombomodulin-Induced Suppression of Peripheral HMGB1-Mediated Allodynia in Mice
Journal of Neuroimmune Pharmacology (2018)
-
Plasminogen/thrombomodulin signaling enhances VEGF expression to promote cutaneous wound healing
Journal of Molecular Medicine (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.