Abstract
We investigated the association between mean platelet volume (MPV) and risk of all-cause mortality in Chinese patients with ST-Elevation Myocardial Infarction (STEMI). We enrolled 1836 patients with STEMI in Xuanwu Hospital from January 2008 to December 2013. Based on MPV, patients were categorized into the following groups: <9.5 fL (n = 85), 9.5–11.0 fL (n = 776), 11.1–12.5 fL (n = 811) and >12.5 fL (n = 164), respectively. Mean duration of follow-up was 56.9 months, and 197 patients (10.7%) died during follow-up. All-cause mortality rates were compared between groups. The lowest mortality occurred in patients with MPV between 9.5–11.0 fL, with a multivariable-adjusted hazard ratio (HR) of 1.15(95%CI 0.62–1.50), 1.38(95%CI 1.20–1.68), and 1.72(95%CI 1.41–1.96) in patients with MPV of <.5, 11.1–12.5 and >12.5 fL, respectively. Therefore, increased MPV was associated with all-cause mortality in Chinese patients with STEMI. MPV might be useful as a marker for risk stratification in Chinese patients with STEMI.
Similar content being viewed by others
Introduction
ST-Elevation Myocardial Infarction (STEMI) is a major public health problem and a leading cause of death both in developed and developing countries. The morbidity and mortality of patients with STEMI remain high despite current advances in drug therapy and revascularization techniques such as percutaneous coronary intervention (PCI) and coronary artery bypass graft surgery (CABG). Considering the poor prognosis, useful biomarkers for predicting future cardiovascular events are needed. Circulating platelets are heterogeneous in size, density and reactivity, and changes in these variables are important for the development of acute coronary syndromes (ACS). Mean platelet volume (MPV), a measurement of the variability in size of circulating platelets, has been previously suggested to be an important hematologic variable and indicator of platelet function1.
According to present studies, increased MPV may be associated with poor clinical outcome among survivors of ACS2,3,4,5,6,7,8. Although the underlying biological mechanisms remain unclear, several potential factors have been postulated, such as larger platelets are metabolically and enzymatically more active than smaller platelets. Larger platelets have a greater content of granules, increased thromboxane synthesis, β-thromboglobulin, serotonin release, increased expression of P-selectin, glycoprotein IIb/IIIa and fibrinogen receptors9,10,11,12.
In the present study, we used a retrospective database to investigate the relation between MPV and risk of all-cause mortality in Chinese patients with STEMI.
Results
Baseline Characteristics
1836 patients with STEMI were enrolled in Xuanwu Hospital from January 2008 to December 2013, and categorized into the following four groups: <9.5 fL (n = 85), 9.5–11.0 fL (n = 776), 11.1–12.5 fL (n = 811) and >12.5 fL (n = 164), respectively. Baseline characteristics and treatments were shown in Table 1. Patients with higher MPV were more likely to be older, and had higher triglyceride (TG), low density lipoprotein cholesterol (LDL-C), white blood cell (WBC) and prevalence of prior myocardial infarction, and had reduced platelet levels. No significant differences in coronary revascularization and medications were identified between the groups.
In a multivariable linear regression model, MPV were associated with age, TG, LDL-C, WBC, platelet and prior myocardial infarction (Table 2).
Clinical Outcomes
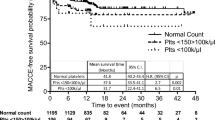
Patients were followed between 2 and 7 years (mean 56.9months). 197 patients (10.7%) died, including 144 deaths from cardiac causes (recurrent myocardial infarction, n = 41; heart failure, n = 75; serious cardiac arrhythmias, n = 23; and sudden death, n = 5) during the follow-up period. Kaplan-Meier curves showed that the lowest cumulative survival rate occurred in patients with MPV >12.5 fL, while the lowest mortality occurred in patients with MPV between 9.5–11.0 fL (p = 0.011; Fig. 1). The results of the Cox proportional hazards model examining the relationship between MPV and all-cause mortality using 9.5–11.0 fL as the reference group. After adjustment for other factors independently associated with mortality, the risk associated with lower MPV was no longer significant. The risk associated with elevated MPV was attenuated but remained statistically significant with increased risk for mortality even for patients with MPV within the normal range (Table 3).
Discussion
The main finding in the present study was that increased MPV was associated with all-cause mortality in Chinese patients with STEMI. The association remained significant even after adjustment for other independent variables.
Platelets are quite heterogeneous blood elements, diverging in terms of size, density and reactivity. The changing of these parameters might be associated with various diseases either as a triggering or propagating factor. In general, the smaller platelet size indicates low production state such as aplastic anemia or myelodystrophic states13, while the larger size indicates a higher destruction rate such as autoimmune related or other acute inflammatory states14. MPV, which is the most accurate measure of the size of platelets, is a simple and easy measurement. A higher MPV has been previously observed in patients with a history of smoking15, diabetes mellitus16, cerebrovascular disease17,18, congestive heart failure19 and in hypertensive patients with evidence of target organ damage20.
Recent studies have showed that a higher MPV might be identified as a marker of increased morbidity and mortality in patients with ACS, especially in STEMI. Martin et al. found that higher MPV values were associated with increased subsequent ischemic events among patients with acute myocardial infarction21. Huczek et al.demonstrated MPV was associated with the no-reflow phenomenon and clinical outcome in patients with STEMI2. Along these lines, Maden et al. reported that MPV was correlated with the presence of infarct-related artery patency (IRA) in a cohort of 351 STEMI patients22. Sezer et al. demonstrated a relationship between MPV values and coronary microvascular injury in patients undergoing PCI23. Rodrigo et al. thought that MPV could predict patency of the IRA before mechanical reperfusion and short-term mortality in patients with STEMI undergoing PCI24. Sarli et al. reckoned that MPV was associated with poor post interventional myocardial blush grade in patients with STEMI6. On the other hand, Yang et al. showed that MPV was a marker of restenosis after percutaneous transluminal coronary angioplasty (PTCA) in patients with stable and unstable angina pectoris25. Additionally, Acar et al. found an association between higher MPV and impaired function of the left ventricle after percutaneous revascularization of the coronaries26. Similarly, we also found that increased MPV was associated with long-term mortality in Chinese patients with STEMI, even after adjustment of other factors.
Although recent studies have showed that increased MPV was an independent risk factor for poor outcomes in cardiovascular disease, the mechanistic links between MPV and poor prognosis in cardiovascular disease have not yet been fully understood. Thrombus formation in the setting of acute myocardial infarction is a complex dynamic process involving both thrombogenesis and thrombolysis and platelets are of pathogenic importance in coronary atherosclerosis and its complications. It has been shown that larger platelets are metabolically and enzymatically more active than smaller ones and aggregate easily27. They express higher levels of prothrombotic substance, thromboxan A2, serotonin, β-thromboglobulin, and procoagulatory surface proteins such as P-selectin and glycoprotein IIIa. These inflammatory makers play a pathogenic role in atherogenesis and cardiovascular disease progression and instability28. An increased MPV decreases the inhibitory effectiveness of prostacyclin I2 (PGI2) on both platelet aggregation and the release reaction29. A shortening of bleeding time and a higher level of P-selectin were previously reported to associate with acute myocardial infarction. Cytokines may possibly trigger the production of larger, more reactive platelets following platelet destruction in peripheral blood. Moreover, elevated levels of CD40 ligands, which are expressed by activated platelets, have been found in atheromatous plaques30. Based on the above Analysis, further studies are needed to clarify the relationship between MPV and different clinical endpoints.
Some limitations in the present study must be considered. This was a single-center, retrospective analysis. Residual confounding factors might thus have affected the results, regardless of the adjusted analysis. Further studies are needed to clarify the mechanisms underlying the relationship between MPV and mortality.
In conclusion, increased MPV was associated with all-cause mortality in Chinese patients with STEMI. MPV might be useful as a marker for risk stratification in Chinese patients with STEMI.
Methods
Study Population
This was a single-center, retrospective analysis. This retrospective study was approved by the Ethics Committee of Xuanwu Hospital, Capital Medical University. The methods were carried out in accordance with the approved guidelines. Since this was a retrospective study, the requirement for informed consent was waived. We enrolled 1836 patients with STEMI in Xuanwu Hospital from January 2008 to December 2013. STEMI was a clinical syndrome defined by characteristic symptoms of myocardial ischemia in association with persistent electrocardiographic (ECG) ST elevation and subsequent release of biomarkers of myocardial necrosis. We excluded patients with any blood, renal, hepatic, autoimmune diseases, malignancy, and the use of thrombolytic drugs within the previous 24 hours. Baseline blood analyses were measured on admission. Demographic data (including age, gender and so on), treatments and comorbidities (hypertension, diabetes, dyslipidemia, prior myocardial infarction and cerebrovascular disease) were also recorded.
Classification of MPV
Baseline MPV was measured on admission using XE-5000 automated hematology analyzer (Sysmex, Kobe, Japan) and the normal range was 9.5–12.5 fL. The majority of patients (n = 1587, 86.5%) had MPV within the normal range, while 85 (4.6%) patients below normal range and 164 (8.9%) above normal range. Considering the small numbers of patients with MPV out of the normal range, the relation between MPV (if it’s divided by tertiles or quartiles) and clinical outcome would be attenuated. It’s more significant when MPV was divided by reference range. Thus, to evaluate the relation between MPV and clinical outcome, the 1836 patients were categorized into the following groups: <9.5 fL (n = 85), 9.5–11.0 fL (n = 776), 11.1–12.5 fL (n = 811) and >12.5 fL(n = 164), respectively.
Clinical Outcome
The end-point of this study was the incidence of all-cause mortality. Cardiac death was defined as death caused by recurrent myocardial infarction, heart failure, serious cardiac arrhythmias and sudden death. Clinical outcome data were collected until 1st November 2015 and mean duration of follow-up was 56.9 months after hospital discharge. Clinical event data were fully collected during the follow-up period for all patients by reviewing the national death registry and by contacting each patient individually and independently reviewing the hospital course for major clinical events if the patient had been rehospitalized.
Statistical Analysis
For baseline characteristics, variables were summarized as percentages for discrete variables and means ± standard deviations for continuous variables. We used χ2 test or analysis of variance, respectively, to test for differences in categorical or continuous factors between different groups of MPV.
Multivariate linear regression was used to determine factors associated with MPV. We used Cox proportional-hazards model with backward selection to examine the association between MPV and clinical outcome. Variables considered for inclusion in the multivariable model included: age, gender, TG, LDL-C, WBC, platelet, coronary revascularization, medication, and history of hypertension, diabetes mellitus, dyslipidemia, prior myocardial infarction and cerebrovascular disease. Survival curves were constructed using the Kaplan–Meier method, and comparisons were made using the log–rank test. Statistical analyses were performed using the SPSS statistical software version 17.0 (Chicago, IL). Differences were considered statistically significant at the 2-sided P < 0.05 level.
Additional Information
How to cite this article: Sun, X.-p. et al. Impact of Mean Platelet Volume on Long-Term Mortality in Chinese Patients with ST-Elevation Myocardial Infarction. Sci. Rep. 6, 21350; doi: 10.1038/srep21350 (2016).
References
Gasparyan, A. Y. et al. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des 17, 47–58, (2011).
Huczek, Z. et al. Mean platelet volume on admission predicts impaired reperfusion and long-term mortality in acute myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol 46, 284–290, (2005).
Roldan V. et al. Mean platelet volume and prognosis in non-ST elevation acute coronary syndrome. Eur Heart J 31, 54–54, (2010).
Goncalves, S. C. et al. Usefulness of mean platelet volume as a biomarker for long-term outcomes after percutaneous coronary intervention. Am J Cardiol 107, 204–209, (2011).
Fabregat-Andres, O. et al. Mean platelet volume is associated with infarct size and microvascular obstruction estimated by cardiac magnetic resonance in ST segment elevation myocardial infarction. Blood Coagul Fibrinolysis 24, 424–427, (2013).
Sarli, B. et al. Mean platelet volume is associated with poor post interventional myocardial blush grade in patients with ST-segment elevation myocardial infarction. Coron Artery Dis 24, 285–289, (2013).
Jakl, M. & Maly, J. Prognostic value of mean platelet volume in patients after acute coronary syndrome. Anadolu Kardiyol Derg 15, 31–32, (2015).
Verdoia, M. et al. Impact of age on mean platelet volume and its relationship with coronary artery disease: a single-centre cohort study. Exp Gerontol 62, 32–36, (2015).
Nording, H. M. et al. Platelets in inflammation and atherogenesis. Front Immunol 6, 98, (2015).
Avramakis, G. et al. Platelets and white blood cell subpopulations among patients with myocardial infarction and unstable angina. Platelets 18, 16–23, (2007).
Avramakis, G. et al. Platelets and white blood cell subpopulations among patients with myocardial infarction and unstable angina. Platelets 18, 16–23, (2007).
Davi, G. & Patrono, C. Platelet activation and atherothrombosis. N Engl J Med 357, 2482–2494, (2007).
Liu, S. et al. Mean platelet volume: a controversial marker of disease activity in Crohn’s disease. Eur J Med Res 17, 27, (2012).
Kurata, Y. et al. Diagnostic value of tests for reticulated platelets, plasma glycocalicin, and thrombopoietin levels for discriminating between hyperdestructive and hypoplastic thrombocytopenia. Am J Clin Pathol 115, 656–664, (2001).
Kario, K., Matsuo, T. & Nakao, K. Cigarette smoking increases the mean platelet volume in elderly patients with risk factors for atherosclerosis. Clin Lab Haematol 14, 281–287 (1992).
Ozder, A. & Eker, H. H. Investigation of mean platelet volume in patients with type 2 diabetes mellitus and in subjects with impaired fasting glucose: a cost-effective tool in primary health care? Int J Clin Exp Med 7, 2292–2297 (2014).
O’Malley, T., Langhorne, P., Elton, R. A. & Stewart, C. Platelet size in stroke patients. Stroke 26, 995–999 (1995).
Mayer, F. J. et al. Mean platelet volume predicts outcome in patients with asymptomatic carotid artery disease. Eur J Clin Invest 44, 22–28, (2014).
Erne, P., Wardle, J., Sanders, K., Lewis, S. M. & Maseri, A. Mean platelet volume and size distribution and their sensitivity to agonists in patients with coronary artery disease and congestive heart failure. Thromb Haemost 59, 259–263 (1988).
Nadar, S. K., Blann, A. D., Kamath, S., Beevers, D. G. & Lip, G. Y. Platelet indexes in relation to target organ damage in high-risk hypertensive patients: a substudy of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT). J Am Coll Cardiol 44, 415–422, (2004).
Martin, J. F., Bath, P. M. & Burr, M. L. Influence of platelet size on outcome after myocardial infarction. Lancet 338, 1409–1411, (1991).
Maden, O. et al. Relationship of admission haematological indices with infarct-related artery patency in patients with acute ST-segment elevation myocardial infarction treated with primary angioplasty. Coron Artery Dis 18, 639–644, (2007).
Sezer, M. et al. Association of haematological indices with the degree of microvascular injury in patients with acute anterior wall myocardial infarction treated with primary percutaneous coronary intervention. Heart 93, 313–318, (2007).
Estevez-Loureiro, R. et al. Mean platelet volume predicts patency of the infarct-related artery before mechanical reperfusion and short-term mortality in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Thromb Res 124, 536–540, (2009).
Yang, A., Pizzulli, L. & Luderitz, B. Mean platelet volume as marker of restenosis after percutaneous transluminal coronary angioplasty in patients with stable and unstable angina pectoris. Thromb Res 117, 371–377, (2006).
Acar, Z. et al. Mean platelet volume on admission is associated with further left ventricular functions in primary PTCA patients. Eur Rev Med Pharmacol Sci 16, 1567–1569 (2012).
Endler, G. et al. Mean platelet volume is an independent risk factor for myocardial infarction but not for coronary artery disease. Br J Haematol 117, 399–404, (2002).
Mathur, A., Robinson, M. S., Cotton, J., Martin, J. F. & Erusalimsky, J. D. Platelet reactivity in acute coronary syndromes: evidence for differences in platelet behaviour between unstable angina and myocardial infarction. Thromb Haemost 85, 989–994, (2001).
Jakubowski, J. A., Adler, B., Thompson, C. B., Valeri, C. R. & Deykin, D. Influence of platelet volume on the ability of prostacyclin to inhibit platelet aggregation and the release reaction. J Lab Clin Med 105, 271–276 (1985).
Geisler, T. & Bhatt, D. L. The role of inflammation in atherothrombosis: current and future strategies of medical treatment. Med Sci Monit 10, RA308–316, (2004).
Acknowledgements
This work was supported by National Program on Key Basic Research Project (973 Program) (2012CB517802).
Author information
Authors and Affiliations
Contributions
X.P.S. and Q.H. contributed to the design of the study; B.Y.L. and W.W.Zh collected the data; J.L. analyzed the data; and X.P.S. wrote the first draft. All the authors contributed to the review and revision of the manuscript, and all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Sun, Xp., Li, By., Li, J. et al. Impact of Mean Platelet Volume on Long-Term Mortality in Chinese Patients with ST-Elevation Myocardial Infarction. Sci Rep 6, 21350 (2016). https://doi.org/10.1038/srep21350
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep21350
This article is cited by
-
Mean platelet volume/platelet count ratio as a predictor of stent thrombosis in patients with ST-segment–elevation myocardial infarction
Irish Journal of Medical Science (1971 -) (2021)
-
Risk stratification based on components of the complete blood count in patients with acute coronary syndrome: A classification and regression tree analysis
Scientific Reports (2018)
-
Increased mean platelet volume (MPV) is an independent predictor of inferior survival in patients with primary and secondary myelofibrosis
International Journal of Hematology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.