Abstract
Oxidative stress and inflammation play crucial role in the pathogenesis of chronic obstructive pulmonary disease (COPD). Most patients with COPD show a poor response to corticosteroids. Hydrogen sulfide (H2S ) has been implicated in the pathogenesis of COPD, but its expression and effects in lung tissue from COPD patients are not clear. In peripheral lung tissue samples from 24 patients, we found that compared with nonsmokers, the protein level of cystathionine-γ-lyase (CSE) was decreased in smokers and COPD patients. CSE mRNA increased but cystathionine-β-synthase (CBS) mRNA decreased in COPD patients. H2S donors increased glutathione and superoxide dismutase in CS exposed U937 cells and inhibited CS-induced TNF-α and IL-8 secretion. Dexamethasone alone had no effect on lipopolysaccharide (LPS) induced TNF-α release by alveolar macrophages from CS exposed rats, however the combination of dexamethasone and H2S donor significantly inhibited TNF-α release. Thus, H2S metabolism is altered in lung tissue of smokers and COPD patients. Supplementation of H2S protects against CS-induced oxidative stress and inflammation in macrophages and H2S on steroid sensitivity deserves further investigation.
Similar content being viewed by others
Introduction
Chronic obstructive pulmonary disease (COPD) is a common chronic inflammatory disease characterized by irreversible progressive airflow limitation. Cigarette smoking is the main risk factor in COPD and results in the imbalance of oxidant and antioxidant and increased airway inflammation in alveolar macrophages1. Oxidative stress prevents steroids from inhibiting activated inflammatory genes by inhibition of histone deacetylase-2 (HDAC2) function and hyperacetylation of glucocorticoid receptors, leading to steroid resistance2,3. The alternative approaches to improve corticosteroid resistance needs to be studied.
Hydrogen sulfide (H2S), previously as a toxic gas, is now believed to be the third member of the gaseotransmitter family4. H2S formation is produced by three enzymes: cystathionine-γ-lyase (CSE), cystathionine-β-synthase (CBS) and 3-mercaptopyruvate sulfurtransferase (3-MST). H2S has been shown to regulate airway tension, oxidative stress, inflammation and fibrosis in various respiratory diseases5.
Our previous work found that patients with acute exacerbation of COPD had lower serum H2S levels than those with stable COPD6. Exogenous NaHS decreases lung pathology score and IL-8 and TNF-α concentrations in lung tissue of cigarette smoke(CS)-exposed rats7. Han et al. also found that NaHS inhibits CS-induced oxidative stress, airway inflammation and the development of emphysema in mice8. Despite animal experiments and in vitro evidence of H2S in the protection of lung diseases, there is no direct evidence of the presence and effect of H2S in the peripheral lung tissue of patients with COPD. The aim of this study was to investigate the expression of endogenous H2S in lung tissue from nonsmokers, smokers and COPD patients and to explore the protective effect of H2S against inflammation and oxidative stress on CS exposed macrophages. In addition, we investigated whether H2S have the potential to enhance corticosteroid sensitivity in macrophages.
Results
Endogenous H2S was changed in smokers and COPD patients
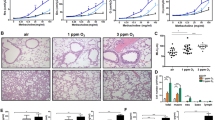
Specimens of peripheral lung tissue were obtained from 11 nonsmokers, 7 smokers who had normal lung function and 6 COPD patients: 2 with stage 1 COPD, 3 with stage 2 COPD and 1 with stage 3 COPD (all of the COPD patients were smokers). We defined COPD and classified the stages of the disease according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) 20149. The mean age of patients was 61 years old and no difference was found among the three groups. Smoking index did not differ between smokers and COPD patients (20(15.4–32.5) vs 37.5(17.5–67.6), P > 0.05). FEV1/FVC and proportion of predicted FEV1 were significantly lower in patients with COPD than in smokers and nonsmokers(P < 0.05, Table 1). Clinical information and patient characteristics were summarized in Table 1. HE staining showed inflammatory cells infiltration in smokers’ lung tissue. Destruction of alveolar walls and enlargement of airspaces were observed in lung tissue of COPD patients (Fig. 1(a)).
Immunohistochemistry showed that CSE was mainly expressed in bronchial and vascular smooth muscle cells and alveolar epithelial cells in nonsmokers’ lung tissue (Fig. 1(b), black arrows). In smokers and COPD patients, CSE expression was decreased. Western blotting showed that the protein level of CSE was decreased in smokers and COPD patients by ~35% and ~39% respectively compared with lung tissue from nonsmokers (P < 0.05, Fig. 2(a,b)). Contrary to the CSE protein expression, the CSE mRNA transcript level was increased in COPD patients compared with nonsmokers and smokers (all P < 0.01, Fig. 2(c)). The CBS mRNA transcript level was increased in smokers compared with nonsmokers (P < 0.05) and decreased in COPD patients compared with nonsmokers (P < 0.05) and smokers (P < 0.01) (Fig. 2(d)). However, there was no significant difference in H2S levels in lung tissue in each group (nonsmokers: 6.46 ± 0.91, smokers: 5.51 ± 0.41, COPD: 5.24 ± 1.35 nmol/mg pro, P > 0.05).
Expression of endogenous H2S in lung tissue.
(a,b) Western blotting analysis of CSE protein expression in lung tissue from nonsmokers, smokers and COPD and relative intensity normalized to the expression of β-actin. (c) CSE mRNA transcripts were measured by Real-time PCR. (d) CBS mRNA transcripts were measured by Real-time PCR. *P < 0.05, **P < 0.01 vs. Nonsmokers; #P < 0.05, ##P < 0.01 vs. Smokers. n = 3–4 patients in each group.
GSH and SOD were decreased in COPD and smokers and H2S increased GSH and SOD in U937 cells
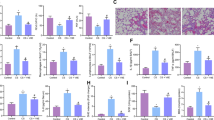
It was showed that GSH and total SOD were decreased in the peripheral lung tissue of smokers and COPD compared with nonsmokers (all P < 0.05, Fig. 3(a,b)). Exposure of U937 to 1%CS for 24 hours caused an increase of intracellular reduced GSH (P < 0.05). Pre-exposure U937 cells to NaHS for 1 hour and then treated with CS for 24 hours further increased intracellular reduced GSH compared with CS alone (P < 0.05, Fig. 3(c)). Intracellular total SOD activity was impaired in CS-treated U937 cells. Pre-treatment U937 cells with NaHS for 1 hour restored SOD activity compared with control (P < 0.05, Fig. 3(d)).
Effects of H2S on intracellular reduced GSH and T-SOD activity.
(a,b) Intracellular reduced GSH and T-SOD activity were reduced in lung tissue of smokers and COPD (n = 4–5 patients in each group). (c,d) NaHS up-regulated intracellular GSH and SOD activity in CS stimulated U937 cells (n = 6–7 experiments). *P < 0.05, **P < 0.01 vs. control; #P < 0.05; ##P < 0.01 vs. CS.
H2S attenuated CS induced inflammation in U937 cells
CS exposure for 18 hours caused a significant increase in TNF-α and IL-8 release from U937 cells (P < 0.05, Fig. 4(a,b)). Co-treatment of CS-exposed U937 cells with GYY4137 (0–500 uM) resulted in a concentration-related inhibition of both TNF-α and IL-8 (Fig. 4(a,b)). Even the lowest concentration of GYY4137 used (i.e., 100 uM) reduced TNF-α formation by 40.3% (P < 0.01) and high concentration of GYY4137 (500 uM) reduced TNF-α and IL-8 formation by 75.4% (P < 0.01) and 85.5% (P < 0.01) respectively, which suggested an anti-inflammatory effect of H2S in this model.
Effects of H2S on the release of TNF-α and IL-8 in U937 cells.
Immediately after CS exposure, U937 cells were treated with increasing concentrations of GYY4137 (100–500 μM). TNF-a (a) and IL-8 (b) were evaluated by enzyme-linked immunosorbent assay. *P < 0.05, **P < 0.01 vs. control; #P < 0.05, ##P < 0.01 vs. CS. n = 4–6 experiments, GYY: GYY4137.
Effect of H2S on the anti-inflammatory effect of dexamethasone
We then investigated whether H2S could further potentiate the anti-inflammatory efficiency of dexamethasone in cells exposed to oxidative stress. U937 cells were pre-exposed to CS and then treated with GYY4137 (100 uM) in the presence of dexamethasone (10−8 M) for 18 hours. Either dexamethasone or GYY4137 could significant suppress the TNF-α release (P < 0.01, Fig. 5(c)). GYY4137 (100 uM) alone failed to suppress IL-8 release (Fig. 5(d)). Combination of GYY4137 and dexamethasone could further reduce the release of TNF-α and IL-8 to some extent (Fig. 5(c,d)).
Effects of H2S on steroid sensitivity.
The expression of HDAC2 protein was decreased in lung tissue of COPD and smokers compared with nonsmokers (a,b). CS-exposed U937 cells were treated with dexamethasone alone or in combination with GYY4137. TNF-a (c) and IL-8 (d) release were evaluated by enzyme-linked immunosorbent assay. (e) TNF-a levels in alveolar macrophages from CS exposed rats were evaluated. *P < 0.05, **P < 0.01 vs. control; #P < 0.05, ##P < 0.01 vs. CS. n = 5–6 experiments, DEX: dexamethasone.
Alveolar macrophages from CS exposed rats were used to further study the interactions between H2S and dexamethasone. SD rats were exposed to cigarette smoke for 4 months and alveolar macrophages were isolated and treated with LPS (10 ng/ml), dexamethasone (10−8 M) or GYY4137 (100 uM). LPS exposure caused a significant increase in TNF-α release (P < 0.05, Fig. 5(e)). Treatment of the LPS exposed macrophages with dexamethasone failed to suppress the TNF-α release. A GYY4137-dexamethasone combination inhibited 47.8% TNF-α release compared with LPS group (P < 0.05, Fig. 5(e)).
Immunohistochemistry showed that HDAC2 was located in nucleus. It was strongly stained in nonsmokers and decreased in smokers and COPD patients (Fig. 6.). The expression of HDAC2 protein was decreased by 37.3% in COPD lung tissue compared with nonsmokers (Fig. 5(a,b)).
Discussion
Previously, we found that patients with acute exacerbation of COPD had lower serum H2S level than those with stable COPD6. Han et al. reported that CSE protein expression was decreased in lung tissue from tobacco smoke exposed mice and pulmonary artery endothelial cells8. This research is the first to explore the different expression of endogenous H2S pathway in lung tissue of nonsmokers, smokers and COPD patients. The protein level of CSE was decreased in COPD patients and smokers. Since all of the COPD patients are smokers in the current study, we believe the protein level of CSE is closely related with the smoking status. However, the mRNA level of CSE was increased in COPD patients while the CBS mRNA level decreased in COPD patients compared with nonsmokers and smokers, suggesting different transcriptional regulation of H2S synthase in COPD patients lung tissues. As a result, H2S levels in lung tissue in each group did not significantly differ. This is similar to those in the literature. In cigarette smoke exposed rats7 or mice8, H2S levels in the lung tissue are not significantly reduced. The H2S level of lung tissue may be influenced by many factors, including different H2S synthase protein expression, mRNA level and enzyme activity. It may also be influenced by smoking because cigarette smoke per se contains H2S. It is worth doing further research on the expression of H2S in more COPD patients and in nonsmoking COPD patients.
The imbalance between oxidants and antioxidants plays an important role in the pathogenesis of COPD. In our research, GSH and SOD levels are reduced in smokers and patients with COPD. H2S has been reported to protect neurons10, vascular smooth muscle cells11 and myocytes12,13 from oxidative stress. Oxidized glutathione (GSSG) are decreased and total antioxidant capacity (T-AOC) increased by NaHS in lung tissue from hypoxic pulmonary hypertensive rats14. In CS exposed mice, the ratio of GSH/GSSG are reduced in the lungs and NaHS increases GSH/GSSG ratio8. In this study, GSH is increased in CS-exposed U937 cells. This increase is probably a compensatory mechanism to offset the marked increase in ROS that are generated upon CS exposure15. Pretreatment U937 cells with NaHS further increases the level of intracellular reduced GSH. The level of total SOD is decreased in CS-exposed U937 cells and NaHS restores the impaired SOD in CS-exposed U937 cells. Therefore, H2S can up-regulate GSH and SOD, which have some implications for antioxidant therapy in COPD.
The role of H2S in inflammation is complex and it has pro-inflammatory effect16,17,18 and meanwhile mounting evidence revealing the anti-inflammatory effect. For instance, recent studies showed that H2S could ameliorate cardiovascular dysfunction by against cecal ligation and puncture (CLP) inducing oxidative stress and inflammation13, improve long-term renal function and reduce long-term inflammation associated with warm renal ischemia and reperfusion injury (IRI)19 and have protective effect in gastrointestinal tract by against inflammation20,21. GYY4137 releases H2S slowly both in vitro and in vivo22. GYY4137 reduces LPS-evoked hypotension and organ damage while reducing plasma cytokine levels in the rat23. In vitro study, GYY4137 inhibits LPS-induced release of pro-inflammatory mediators in macrophages24. In CS-exposed U937 cells, we find a similar anti-inflammatory effect of GYY4137 to inhibit CS induced TNF-α and IL-8. The anti-inflammatory effect of H2S may be due to inhibit transcription factors (such as NF-κB or AP-1) activation according to previous reports25,26,27.
Glucocorticoid resistance is known to occur in COPD and severe asthma due to increased oxidative stress28,29,30. Sulforaphane, as Nrf2 activator, is able to restore corticosteroid resistance in alveolar macrophages from patients with COPD31. Studies showed that HDAC2 is associated with corticosteroid sensitivity via activation of the phosphoinositide 3 kinase delta (PI3K-δ)32,33. Since H2S has anti-oxidant and anti-inflammatory effect, we try to investigate whether H2S could enhance corticosteroid sensitivity. In CS exposed U937 cells, when compared with dexamethasone alone, combination of GYY4137 and dexamethasone could further reduce the release of TNF-α and IL-8 to some extent. Alveolar macrophages from CS exposed rats were cultured to further study the effect of H2S on steroid sensitivity since this model is more similar to alveolar macrophages from COPD patients. Dexamethasone at low concentration fails to suppress LPS-stimulated-TNF-α release. Co-incubation of GYY4137 and dexamethasone significantly reduces TNF-α release. Immunohistochemistry showed that HDAC2 was strongly stained in nonsmokers and decreased in smokers and COPD patients. The expression of HDAC2 protein was decreased in COPD lung tissue compared with nonsmokers, which is similar to the previous studies. This preliminary study suggests that H2S may have the potential to enhance the anti-inflammatory effect of dexamethasone but still needs further investigation.
H2S as a novel signal molecule like nitric oxide (NO) and carbon monoxide (CO), plays a pivotal role in some physiological and pathological conditions. Our research demonstrate that exogenous supplemented H2S could attenuate cigarette smoking induced inflammation, oxidative stress and improve the response to corticosteroids as well. In clinical COPD patients, especially who resist corticosteroid is a difficult problem to solve. We deduce that clinically used H2S may be a novel therapeutic strategy in patients with COPD and provides a novel approach to reversing corticosteroid insensitivity in COPD with high translational potential.
There are some limitations of the study. Firstly, the number of lung tissue samples were small and do not include non-smoking COPD patients. In the future study, we may recruit COPD patients in different GOLD stages and non-smoking COPD patients. Secondly, the mechanism of H2S on other additional inflammatory markers demand further investigation. COPD involves a complex inflammatory process34. In additon to TNF-α and IL-8, there are other inflammatory markers as well, for instance, LTB4, MCP-1, CXCR2, CXCR3, IL-1, MMP-9 and so on35. In this research we chose our interested inflammatory markers TNF-α and IL-8 because our previous research found that H2S decrease their concentrations in lung tissue of cigarette smoke exposed rats7. Moreover, TNF-α and IL-8 also reported play a vital role in corticosteroid resistance patients with COPD36,37,38.
In summary, the present study showed the altered H2S metabolism in smokers and patients with COPD. Supplementation of H2S increased GSH and SOD levels and inhibited IL-8 and TNF-α secretion in CS exposed macrophages. The combination of dexamethasone and H2S donor significantly inhibited TNF-α release. The effect of H2S on steroid sensitivity deserves further investigation.
Methods
Patients
Human lung tissue samples were obtained from patients undergoing thoracic surgery for removal of a primary lung tumor from the Department of Thoracic Surgery, Peking University Third Hospital, Beijing, China from Apr to Aug in 2012. We kept normal lung tissue from a non-involved segment, remote from the solitary lesion. All tissue samples were stored in −80 °C and treated at the same time with a unified approach. The protocol was approved by the Ethics Committee of Peking University Third Hospital, approval number IRB00006761-2012029. Informed written consent was obtained from each participant. COPD was diagnosed according to the criteria recommended by the Chinese Medical Society39.
Immunohistochemistry
For CSE and HDAC2 immunohistochemical analysis of human pulmonary tissue, specimens were fixed, embedded in paraffin, cut into sections (4–6 μm) and stained with haematoxylin, as reported previously(7). The sections were incubated with mouse anti-human CSE antibody (1:25; Abnova) or HDAC2 antibody (1:50; Cell Signaling Technology) for 24 h at 4 °C. Anti-goat secondary antibody (1:100; ZSGB-BIO) conjugated with DAB was used for detection. Non-immune IgG isotype was used as a negative control.
Isolation and culture of alveolar macrophages
Sprague–Dawley rats were exposed to cigarette smoke for 4 h/day, 6 days/week for 4 months using a dynamic smoke exposure box (diameter 700 mm, Tianjin Hope Corp., Tianjin, China). Bronchoalveolar lavage (BAL) was collected carefully and centrifuged 500 g for 5 min. Alveolar macrophages were isolated by plastic adhesion and cells (105/well) were incubated in 96-well plates in the presence or absence of LPS, dexamethasone and GYY4137(morpholin-4-ium 4 methoxyphenyl (morpholino) phosphinodithioate, Cayman chemical) for 18 hours. All animal care and experimental protocals were in compliance with the PR China Animal Management Rule and the Third Hospital, Peking University Guide for the Care and Use of Laboratory Animals.
U937 cell culture and treatments
The human monocytic cell line U937 was purchased from Cell Resource Center, Chinese Academy of Medical Science. U937 cells were maintained in complete growth medium (RPMI 1640) supplemented with 10% fetal bovine serum (FBS, Gibco), 2 mM L-glutamine, 100 U/ml penicillin and 100 mg/ml streptomycin at 37 °C in a humidified atmosphere with 5% CO2. U937 were differentiated into an adherent “macrophage-like” morphology by exposure to PMA (30 ng/ml, Sigma) for 48 hours. After differentiation, cells were starved overnight and then subjected to oxidative stress for 24 hours using CS (1%) with/without H2S donor (NaHS or GYY4137). Cell toxicity was monitored by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay.
Preparations of cigarette smoke extract
Ten percent CS was prepared using two full-strength commercial “Dubao” cigarettes with filters removed which were combusted through a modified 60-mL syringe apparatus into 20 mL of RPMI 1640 medium, as previously described40.
Measurement of H2S content in lung tissue
The H2S content in lung tissue was measured as described previously7. It was analyzed by use of sulfide-sensitive electrodes (PXS-270; Shanghai) and the H2S concentration was expressed as nanomoles per milligram protein.
The measurement of GSH and SOD
Total intracellular reduced GSH and SOD were measured by assay kits (Boster, Inc., Wuhan, China) according to the manufacturer’s instructions.
Enzyme-linked immunosorbent assay
IL-8 and TNF-a levels were assayed in culture supernatant samples by using commercially available enzyme-linked immunosorbent assay kits (Boster, Inc., Wuhan, China) according to the manufacturer’s protocol.
Western blotting analysis of CSE and HDAC2
Protein extracts from lung tissue were resolved by 10% SDS-PAGE and then transferred to a nitrocellulose membrane. The membranes were incubated with primary antibody (CSE or HDAC2, dilution 1:1000) and fluorescein-linked secondary antibody (dilution 1:2000) and then detected by enhanced chemiluminescence method. The protein contents were normalized to that of beta-actin.
Real-time PCR
Real-time PCR was performed as previously described41. The forward and reverse PCR primers (human) were CSE_F: 5′-TTCAGGTTTAGCAGCCACTGT-3′, CSE_R: 5′-CCTCCATACACATCATCCATACA-3′. CBS_F: 5′-CTGAACTGTCAGCACCATCTGT-3′ CBS_R: 5′-CTCCTTGGCTTCCTTATCCTCT-3′ Relative quantification of different transcripts was determined by the 2−ΔΔCt method, using glyceraldehydes-3-phosphate dehydrogenase (GAPDH) as an endogenous control and with normalization to the control group.
Statistics
The data are expressed as mean ± SD (for normally distributed data) or median (for non-normally distributed data). One-way ANOVA was used to compare more than 2 groups and when significant (P < 0.05), the Tukey HSD test was used to test for differences between groups. A P < 0.05 was considered statistically significant.
Additional Information
How to cite this article: Sun, Y. et al. Metabolic changes of H2S in smokers and patients of COPD which might involve in inflammation, oxidative stress and steroid sensitivity. Sci. Rep. 5, 14971; doi: 10.1038/srep14971 (2015).
References
Barnes, P. J. Alveolar macrophages in chronic obstructive pulmonary disease (COPD). Cellular and molecular biology (Noisy-le-Grand, France). 50 Online Pub, Ol627–37 (2004).
Barnes, P. J. New concepts in chronic obstructive pulmonary disease. Annual review of medicine. 54, 113–29 (2003).
Barnes, P. J., Ito, K. & Adcock, I. M. Corticosteroid resistance in chronic obstructive pulmonary disease: inactivation of histone deacetylase. Lancet. 363, 731–3 (2004).
Wang, R. Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 16, 1792–8 (2002).
Chen, Y. & Wang, R. The message in the air: hydrogen sulfide metabolism in chronic respiratory diseases. Respiratory physiology & neurobiology. 184, 130–8 (2012).
Chen, Y. H. et al. Endogenous hydrogen sulfide in patients with COPD. Chest. 128, 3205–11 (2005).
Chen, Y. H. et al. Involvement of endogenous hydrogen sulfide in cigarette smoke-induced changes in airway responsiveness and inflammation of rat lung. Cytokine. 53, 334–41 (2011).
Han, W., Dong, Z., Dimitropoulou, C. & Su, Y. Hydrogen sulfide ameliorates tobacco smoke-induced oxidative stress and emphysema in mice. Antioxidants & redox signaling. 15, 2121–34 (2011).
Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for Diagnosis, Management and Prevention of COPD. Available from www.goldcopd.org 2014.
Kimura, Y. & Kimura, H. Hydrogen sulfide protects neurons from oxidative stress. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 18, 1165–7 (2004).
Yan, S. K. et al. Effects of hydrogen sulfide on homocysteine-induced oxidative stress in vascular smooth muscle cells. Biochemical and biophysical research communications. 351, 485–91 (2006).
Geng, B. et al. Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochemical and biophysical research communications. 318, 756–63 (2004).
Abdelrahman, R. S., El-Awady, M. S., Nader, M. A. & Ammar, E. M. Hydrogen sulfide ameliorates cardiovascular dysfunction induced by cecal ligation and puncture in rats. Human & experimental toxicology. 10.1177/0960327114564794 (2015).
Wei, H. L., Zhang, C. Y., Jin, H. F., Tang, C. S. & Du, J. B. Hydrogen sulfide regulates lung tissue-oxidized glutathione and total antioxidant capacity in hypoxic pulmonary hypertensive rats. Acta pharmacologica Sinica. 29, 670–9 (2008).
Tollefson, A. K. et al. Endogenous enzymes (NOX and ECSOD) regulate smoke-induced oxidative stress. Free radical biology & medicine. 49, 1937–46 (2010).
Muniraj, N. et al. Hydrogen sulfide acts as a pro-inflammatory mediator in rheumatic disease. International journal of rheumatic diseases. 10.1111/1756-185x.12472 (2014).
Chi, X. P., Ouyang, X. Y. & Wang, Y. X. Hydrogen sulfide synergistically upregulates Porphyromonas gingivalis lipopolysaccharide-induced expression of IL-6 and IL-8 via NF-kappaB signalling in periodontal fibroblasts. Arch Oral Biol. 59, 954–61 (2014).
Li, L., Fox, B. et al. The complex effects of the slow-releasing hydrogen sulfide donor GYY4137 in a model of acute joint inflammation and in human cartilage cells. Journal of cellular and molecular medicine. 17, 365–76 (2013).
Lobb, I. et al. Hydrogen sulfide treatment ameliorates long-term renal dysfunction resulting from prolonged warm renal ischemia-reperfusion injury. Can Urol Assoc J. 8, E413–8 (2014).
Motta, J. P. et al. Hydrogen Sulfide Protects from Colitis and Restores Intestinal Microbiota Biofilm and Mucus Production. Inflamm Bowel Dis. 21, 1006–17 (2015).
Magierowski, M. et al. Endogenous Prostaglandins and Afferent Sensory Nerves in Gastroprotective Effect of Hydrogen Sulfide against Stress-Induced Gastric Lesions. PloS one. 10, e0118972 (2015).
Li, L. et al. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation. 117, 2351–60 (2008).
Li, L., Salto-Tellez, M., Tan, C. H., Whiteman, M. & Moore, P. K. GYY4137, a novel hydrogen sulfide-releasing molecule, protects against endotoxic shock in the rat. Free radical biology & medicine. 47, 103–13 (2009).
Whiteman, M. et al. The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. Antioxidants & redox signaling. 12, 1147–54 (2010).
Oh, G. S. et al. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-kappaB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free radical biology & medicine. 41, 106–19 (2006).
Li, L. et al. Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative. Free radical biology & medicine. 42, 706–19 (2007).
Florian, B. et al. Long-term hypothermia reduces infarct volume in aged rats after focal ischemia. Neuroscience letters. 438, 180–5 (2008).
Hakim, A., Barnes, P. J., Adcock, I. M. & Usmani, O. S. Importin-7 mediates glucocorticoid receptor nuclear import and is impaired by oxidative stress, leading to glucocorticoid insensitivity. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 27, 4510–9 (2013).
Chung, K. F. & Marwick, J. A. Molecular mechanisms of oxidative stress in airways and lungs with reference to asthma and chronic obstructive pulmonary disease. Annals of the New York Academy of Sciences. 1203, 85–91 (2010).
Barnes, P. J. Mechanisms and resistance in glucocorticoid control of inflammation. The Journal of steroid biochemistry and molecular biology. 120, 76–85 (2010).
Malhotra, D. et al. Denitrosylation of HDAC2 by targeting Nrf2 restores glucocorticosteroid sensitivity in macrophages from COPD patients. The Journal of clinical investigation. 121, 4289–302 (2011).
Kobayashi, Y. et al. A novel macrolide/fluoroketolide, solithromycin (CEM-101), reverses corticosteroid insensitivity via phosphoinositide 3-kinase pathway inhibition. British journal of pharmacology. 169, 1024–34 (2013).
To, Y. et al. Targeting phosphoinositide-3-kinase-delta with theophylline reverses corticosteroid insensitivity in chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 182, 897–904 (2010).
Aina Noguera, C. G. et al. An investigation of the resolution of inflammation (catabasis) in COPD. Respiratory research. 13, 10.1186/1465-9921-13-101 (2012).
Barnes, P. J. Alveolar macrophages as orchestrators of COPD. Copd. 1, 59–70 (2004).
Chana, K. K., Fenwick, P. S., Nicholson, A. G., Barnes, P. J. & Donnelly, L. E. Identification of a distinct glucocorticosteroid-insensitive pulmonary macrophage phenotype in patients with chronic obstructive pulmonary disease. The Journal of allergy and clinical immunology. 133, 207–16 e1-11 (2014).
Armstrong, J., Sargent, C. & Singh, D. Glucocorticoid sensitivity of lipopolysaccharide-stimulated chronic obstructive pulmonary disease alveolar macrophages. Clin Exp Immunol. 158, 74–83 (2009).
Jurgen Knobloch, H. H., David, J., Katja, U. & Andrea, K. Resveratrol Impairs the Release of Steroid-resistant Cytokines from Bacterial Endotoxin-Exposed Alveolar Macrophages in Chronic Obstructive Pulmonary Disease. Basic & Clinical Pharmacology & Toxicology. 109, 138–143 (2011).
CMA guideline for the management of chronic obstructive pulmonary disease. Chin J Tuber Respir. 36, 255–264 (2013).
Walters, M. J. et al. Cigarette smoke activates human monocytes by an oxidant-AP-1 signaling pathway: implications for steroid resistance. Molecular pharmacology. 68, 1343–53 (2005).
Cai, Y. et al. Intermedin inhibits vascular calcification by increasing the level of matrix gamma-carboxyglutamic acid protein. Cardiovascular research. 85, 864–73 (2010).
Acknowledgements
The authors thank Ying-Mei Zhang for kindly providing some U937 cells and Lei Zhao, Yan-Jing He for technical assistance. Funding: This study was supported by National Nature Science Foundation of China (No. 81170012 and 81370141), The Research Special Fund for Public Welfare Industry of Health (No. 201002008) and Capital Medicine Development Fund (2011-1004-01).
Author information
Authors and Affiliations
Contributions
Y.S., K.-Y.W., M.-X.L., W.H., J.-R.C., C.-C.L., F.L., Y.-F.Q., R.W. and Y.-H.C., were involved in the conception and design of experiments, analyzing the data and writing the manuscript. Y.S., J.-R.C., C.-C.L. and F.L., performed the experiments. Y.-H.C., K.-Y.W. and W.H. obtained informed consent and collected human samples. Y.S., K.-Y.W. and M.-X.L. analyzed the data and wrote the manuscript and contributed equally to the manuscript. Y.-F.Q. is the senior author providing the experiment guidance. All authors read and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Sun, Y., Wang, K., Li, MX. et al. Metabolic changes of H2S in smokers and patients of COPD which might involve in inflammation, oxidative stress and steroid sensitivity. Sci Rep 5, 14971 (2015). https://doi.org/10.1038/srep14971
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep14971
This article is cited by
-
Cystine rather than cysteine is the preferred substrate for β-elimination by cystathionine γ-lyase: implications for dietary methionine restriction
GeroScience (2023)
-
H2S in acute lung injury: a therapeutic dead end(?)
Intensive Care Medicine Experimental (2020)
-
Cystathionine γ-lyase deficiency enhances airway reactivity and viral-induced disease in mice exposed to side-stream tobacco smoke
Pediatric Research (2019)
-
Interaction of the hydrogen sulfide system with the oxytocin system in the injured mouse heart
Intensive Care Medicine Experimental (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.