Abstract
Prophages of Helicobacter pylori, a bacterium known to co-evolve in the stomach of its human host, were recently identified. However, their role in the diversity of H. pylori strains is unknown. We demonstrate here and for the first time that the diversity of the prophage genes offers the ability to distinguish between European populations and that H. pylori prophages and their host bacteria share a complex evolutionary history. By comparing the phylogenetic trees of two prophage genes (integrase and holin) and the multilocus sequence typing (MLST)-based data obtained for seven housekeeping genes, we observed that the majority of the strains belong to the same phylogeographic group in both trees. Furthermore, we found that the Bayesian analysis of the population structure of the prophage genes identified two H. pylori European populations, hpNEurope and hpSWEurope, while the MLST sequences identified one European population, hpEurope. The population structure analysis of H. pylori prophages was even more discriminative than the traditional MLST-based method for the European population. Prophages are new players to be considered not only to show the diversity of H. pylori strains but also to more sharply define human populations.
Similar content being viewed by others
Introduction
To discover and follow human migrations, the use of information from genomes of human pathogens and commensals which present a phylogeographic division, may offer additional insight into the corresponding human genome sequences themselves. Helicobacter pylori and man co-evolved together, since they went ‘out of Africa’1,2. This bacterium colonizes the human stomach of more than half of the human population. The infection is not without a clinical impact and although the majority of the human hosts do not present any symptom, gastritis is present in all cases. Complications of gastritis include peptic ulcer disease and, in rare cases, gastric adenocarcinoma and mucosa associated lymphoid tissue (MALT) lymphoma3.
The co-evolution of the bacteria with the human host is verified by phylogenetic analysis which produces bacterial clusters according to the geographic origin of the bacterium and its host (reviewed in4,5). Currently, 7 H. pylori bacterial populations have been described, following MLST analysis of 7 housekeeping genes2,6 but only one European population, hpEurope, is considered here.
Bacteriophages (phages) are viruses which infect bacteria. Lytic phages have the property to lyse the bacterial cells and release the phage progeny, while lysogenic or temperate phages may go either through a lytic cycle or the phage genome may be integrated in the bacterial genome, constituting a prophage. Temperate phages contribute to the evolution of most bacteria, by promoting the transduction of various genes involved in virulence, fitness and antibiotic resistance7. Even if in the human gut there are about 109 virus-like particles per gram of faeces8, reports on H. pylori phages are still sparse. Moreover, the first H. pylori prophage was described almost 30 years after the discovery of H. pylori. During that long period, H. pylori was considered as a bacterium without prophages. Indeed the first H. pylori genomes sequenced did not reveal the presence of prophages, until the identification of a remnant prophage integrated into the genome of H. pylori strain B389, confirmed later by the discovery of a larger prophage in strain B4510 and followed by other publications11,12. The prevalence of H. pylori prophages, inferred by the presence of the phage integrase gene, is estimated to be approximately 20%10 in H. pylori strains.
Prophages and bacteria are linked by a long history of co-evolution, but the genetic dimension of this co-evolution cannot be defined at present7. Indeed, a phylogenetic analysis of the integrase gene sequences present in H. pylori prophages revealed a strong phylogeographic signal within the phage integrase gene, which was in agreement with a model of co-evolution between the virus and its bacterial host. The presence of prophages in other non-pylori Helicobacter species, such as Helicobacter acinonychis13, Helicobacter felis14, or Helicobacter bizzozeronii15 which share homology with H. pylori prophages points to a prophage acquisition before speciation. The presence of remnant prophages (prophage fragments) in H. pylori strains9 and in non-pylori Helicobacters16, indicates a prophage decay during the complex interaction between H. pylori and the prophage. However, a model in which H. pylori strains from different geographical regions may have been infected by distinct phage lineages after the geographic separation of the bacterial host is also feasible10.
In order to understand if the prophage population structure coincides with its host structure, a group of 870 H. pylori strains were screened for the presence of prophages. Among them, 41 strains were positive for two prophage genes.
The H. pylori genomes and whole-genome shotgun (WGS) contigs databases were also Blast for the presence of the integrase and holin genes, allowing the identification of 22 H. pylori genomes which were also included in the study. These strains were selected and typed using the MLST method and a newly implemented method here designated as prophage sequence typing (PST), which targets the two prophage genes (integrase and holin) of H. pylori. A Bayesian clustering analysis was used for the identification of distinct genetic populations complemented by phylogenetic analysis, in order to determine the population structure of the host strains and their prophages. These approaches highlighted the diversity in the population structure of the H. pylori prophages. The present study demonstrates that the population structure analysis of H. pylori prophages discriminates between two different European populations while the traditional MLST-based method only distinguishes one.
Results and Discussion
Identification of H. pylori strains carrying prophages
The presence of prophages in H. pylori strains was confirmed by screening for 1) the integrase gene responsible for the integration of the phage genome into the bacterial chromosome and 2) the holin gene involved in cell lysis when a lytic cycle occurs7. The integrase gene was previously shown to be a good marker for the presence of prophages10 and is usually placed at the left end, while the holin gene, is part of the lytic cassette and is usually placed on the right side. The presence of both genes may be indicative of intact prophages. Prophage sequences are highly heterogeneous, which can lead to false negative and positive PCR results. To overcome this problem, all PCR products were sequenced. The prophage sequences employed in the present study are available at GenBank (No: KM275873 to KM275935). The remaining integrase gene sequences were described previously10.
Due to their diversity it is possible that some prophages were not identified with these primer pairs. However, in light of the limited number of prophage sequences currently available, this approach was considered to be acceptable. Among the 870 H. pylori DNAs originally constituted, 161 (18.5%) were positive for the integrase gene and 41 (4.7%) for both the integrase and holin genes (Supplementary Table S2, strains 1 to 41).
The software for the identification of prophages17, applied to 53 H. pylori complete genomes listed at REBASE genomes18 in October 2014, revealed the presence of prophages in 18.9% (10/53) of the genomes. Only 5.6% (3/53) of these prophage positive genomes carried both the integrase and holin genes. These percentages are similar to those described in the present study. Moreover, a Blastn analysis19 of the integrase and holin genes using the nucleotide and the whole-genome shotgun contig databases allowed the identification of 22 genomes carrying these genes that were included in the analysis (Supplementary Table S1).
H. pylori-prophage population structure
After performing MLST using seven housekeeping genes, a phylogenetic tree was constructed including 741 concatenated sequences available at PubMLST for H. pylori (http://pubmlst.org/helicobacter/) and originally described by Falush et al.1 and Linz et al.2 plus 41 sequences from strains presenting the prophage genes and the 22 genomes previously selected (see above) (Fig. 1). The DNA sequences of the seven MLST genes are available at PubMLST (http://pubmlst.org/helicobacter/) with the profile numbers 2851 to 2886. The analysis shows that the strains included in the present study plus the genomes of H. pylori from public databases are dispersed throughout the major H. pylori populations.
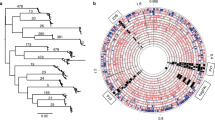
Neighbour-joining H. pylori MLST population tree of 804 strains.
741 strains from PubMLST (corresponding to initial studies by Falush et al.1 and Linz et al.2) and 63 strains from the present study. (A) The major H. pylori populations (six populations and three sub-populations) were identified according to the assigned population available at PubMLST. (B) Identification and position in the tree of the 41 strains and 22 genomes carrying the prophage integrase and holin genes.
For the Structure 2.3.4. analysis of the seven MLST genes of all 804 strains (741 from PubMLST, 41 presenting the prophage genes in the initial collection and 22 from public databases), the best posterior probabilities were achieved for K ≥ 6, but K > 6 produced inconsistent populations or hypothetical populations with no assigned individuals. Thus K = 6 was considered the best value. The major populations defined were consistent with prior assignments2 and were used to classify the 63 strains included in this study (Supplementary Table S2). Briefly, concerning the 41 selected strains, most were classified according to their country of origin (28 hpEurope strains, 9 hpAfrica1 strains and 4 hpEastAsia strains), with a few exceptions: one strain from France was classified as hpEastAsia and one strain from France and three strains from Portugal were classified as hpWAfrica or hpNEAfrica (Supplementary Table S2). Similar results were found for the 22 H. pylori genomes identified in public databases, i.e., when information was available, the population structure corresponded to the country of isolation, especially considering the diversity of isolates brought to the New World by non-Amerindian hosts20.
In the case of the Bayesian analyses of the seven MLST genes for the group of 63 strains carrying the prophage genes, the best posterior probability was achieved for K = 4, dividing the group into four populations: hpEurope (30 strains), hpEastAsia (10 strains), hpAfrica1 (22 strains) and hpAfrica2 (1 strain) (Fig. 2B). Using K = 5, a further hypothetical population with no assigned individuals was detected and thus K = 4 fits these data the best. The same methodology was then applied to the prophage data. Surprisingly it produced four populations, since K = 4 presented the best posterior probability, showing two European populations. These four populations identified by PST (Fig. 2A) are hpEastAsia (11 strains), hpAfrica1 (23 strains) and two new European populations, hpSWEurope (13 strains) and hpNEurope (16 strains). The single strain comprising the hpAfrica2 population carries a prophage classified as hpNEurope. Briefly, the prophage sequences distinguished four populations with the parameters used and the MLST sequences distinguished only three (not taking into account the hpAfrica2 strain, which appears to be a mosaic strain for the prophage sequence as explained below).
Distruct plot of Bayesian population assignments using STRUCTURE 2.3.4. and an admixture model (K = 4) for the 63 strains carrying prophages.
(A) Distruct plot obtained with the prophage gene sequences (PST) and (B) with the sequences of seven housekeeping genes (MLST). Each bacterial isolate is depicted by a thin vertical line, which is divided into K coloured segments representing the membership coefficients in each cluster.
A tree for the 63 strains according to MLST is presented in Fig. 3B, including their population structure obtained after applying Structure 2.3.4.21,22,23 software. In both figures (Figs 1 and 3B.) there is a continuous distribution of the ancestry proportions, suggesting admixture due to recombination2.
(A) Neighbour-joining tree (Kimura 2-parameter) of 63 concatenated prophage sequences from H. pylori strains carrying prophages colour-coded according to the population assigment by STRUCTURE using the prophage genes. (B) Neighbour-joining tree (Kimura 2-parameter) of 63 concatenated sequences obtained after MLST of H. pylori strains carrying prophages and their attributed population structure after STRUCTURE analysis.
A phylogenetic tree using the neighbour-joining method and the Kimura 2-parameter for the concatenated prophage genes showed that strains cluster according to their population assigned by Structure 2.3.4.21,22,23, in a continuous distribution (Fig. 3A). However, this distribution (Fig. 3A), is not as continuous as that observed in the H. pylori tree produced with the concatenated sequences of the seven housekeeping genes (Fig. 3B), revealing an increased diversity in the prophage gene sequences compared to the housekeeping genes. In fact, when only the sequences of the 63 strains were considered, among the 3,406 base pairs of the housekeeping genes, 824 positions were polymorphic, while among the 754 base pairs of the two prophage genes, 445 positions were polymorphic. The increased variability of H. pylori compared to its human host has been described and has allowed H. pylori to be used as a tool to trace human populations1. The prophage sequences could also be viewed as a tracer of human populations, allowing us to discriminate between two different European populations.
Falush et al. described the existence of two ancient European populations, AE1 and AE2 and considered that, according to MLST data, European isolates are recombinants of AE1 and AE2. AE1 is described as being higher in number in Northern Europe and Ladakh, while AE2 is higher in Southern Europe (Spain), Sudan and Israel1. AE1 is speculated to have arisen in Central Asia, while AE2 would had split from its sister lineage hpAfrica1, which then came in contact in Europe, forming the hybrid population hpEurope24. The European strains included in the present study confirm the mosaicism of small multiple chromosomal chunks observed after analysis of the seven housekeeping genes (Supplementary Fig. S1B). The genes included in the MLST for H. pylori are therefore not able to discriminate between current European populations, which have been attributed to multiple and complex migratory events that occurred over the European continent since its first colonization by modern men. However, considering the MLST analysis, a closer inspection of Fig. 3B shows that the hpNEurope strains from Fig. 3A cluster mostly in the right branch of the tree (the rare exceptions were hpAfrica2 strain Za-SA160A and two other mosaic strains Us-HpP4-G and Za-SA156A), whereas all hpSWEurope strains in Fig. 3A were found in the left branch of Fig. 3B. This was also evident in Fig. S1, where the hpSWEurope strains (in Table S2 with the numbers: 9, 21, 27, 29, 33, 35, 38, 39, 41, 44, 61, 62, 63) look quite different from the hpNEurope strains (in Table S2 with the numbers: 1, 2, 3, 4, 5, 6, 22, 23, 24, 42, 43, 45, 46, 47, 48, 49) according to MLST. Indeed, in Fig. S1 most of the strains classified as hpSWEurope by prophage typing are mosaics of hpEurope and hpAfrica1, while most of the strains considered hpNEurope according to prophage sequences, are in fact MLST mixtures of hpEurope and hpAsia2. Taken together these observations appear to indicate that the MLST sequences can weakly distinguish between H. pylori isolates from the north and southwest of Europe.
Surprisingly the analysis of the population structure according to the prophage genes of H. pylori discriminates between four populations, two of them from Europe showing little evidence of mosaicism between the Northern and Southwestern populations (Fig. 2). The primitive peoples that inhabited Europe, with their different migratory roots and languages (Germanic and Romance) could explain the existence of different European populations. The analysis of the prophage sequences can thus be a useful addition to MLST data to enable a discrimination between European populations.
Considering the mosaic structure of the European isolates according to MLST classification, an 84.1% concordance between the bacteria and the prophage genome population structure was observed (Supplementary Table S3). The discordant cases concern six strains (one French, three Portuguese, one South African and one North American), classified as hpAfrica1 according to bacterial genes and as hpSWEurope according to prophage genes; two strains (one French and one Portuguese) were both classified as hpEurope according to MLST and as hpAfrica according to PST; one Malaysian strain was classified as hpAsia2 by MLST and hpNEurope by PST (this particular strain appears to be a mosaic for MLST, i.e. a mixture between hpAsia2 and hpEastAsia and a mosaic for PST, i.e. a mixture between hpNEurope and hpAfrica); and finally the single South African strain classified as hpAfrica2 by MLST and as hpNEurope by PST (this strain appears to be a mosaic for PST, a mixture of hpNEurope and hpAfrica). For the cases with no evidence of mosaicism, it is tempting to speculate that the prophage may have been recently acquired by horizontal gene transfer. Other isolates, classified differently according to one system or the other, were indeed mosaics. In fact, for these isolates the bacterial MLST or the PST showed evidence of a recombination with the population attributed by the other method and these cases were thus considered as concordant classification. For instance, the strain Pt-5771-G showed a mosaicism between hpEurope and hpAfrica1 for the MLST genes and was classified as hpEurope by MLST and hpAfrica by PST (Supplementary Table S3). Taking into consideration the ancestral European populations, hpNEurope is most closely related to AE1 and hpSWEurope to AE2. Interestingly, most of the cases of discordant classification by MLST and PST concern exchanges between 1) MLST hpAfrica1 and PST hpSWEurope which appear to be closely related to AE2 which is a split from its sister lineage hpAfrica1 or 2) MLST hpAsia2 and PST hpNEurope, closely related to AE1, which have probably arisen from Central Asia. The segregation observed in European populations is in accordance with recent human genetic data which can place individuals in different European localizations using 40,000–130,000 single nucleotide polymorphisms (SNP)25,26. Moreover, the segregation of European populations observed in the prophage gene analysis appears to be in agreement with the most recent data concerning ancestral populations of Europe, influenced by the ancient people of Western Europe, Northern Eurasia and the Near East27.
In summary, our results show the existence of different European populations which could be traced using dormant phages of H. pylori. Thus PST is able to differentiate individual populations within the European population, which is in agreement with recent human genome-wide analysis favouring the hypothesis that present-day European genetic diversity is strongly correlated with geography27,28.
Methods
H. pylori strains
Genomic DNA samples from 870 H. pylori strains, isolated from patients living in different continents (692 were from Europe, 117 from Asia, 1 from the USA and 60 from Africa) and suffering from different diseases (249 peptic ulcer, 450 gastritis, 77 adenocarcinoma and 65 MALT lymphoma; information concerning the associated disease was lacking for 29 strains) were collected. These DNAs were extracted using standard protocols after culture of H. pylori strains belonging to the collection of the French National Reference Centre for Campylobacters and Helicobacters (F. Mégraud and P. Lehours, Bordeaux, France); the Department of Microbiology, Tumor and Cell Biology, Karolinska Institute (Lars Engstrand); the Klinikum Rechts Der Isar II, Medical Department, Technische Universität, Munich, Germany (M. Gerhard); the Department of Medicine-Gastroenterology, Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine, Houston, TX, USA (Y. Yamaoka); the Department of Infectious Diseases, National Institute of Health, Lisbon, Portugal (M. Oleastro); the Rabin Medical Center – Beilinson Hospital, Petah Tikva, Israel (T.T. Perets and Y. Niv); and the Pasteur Institute of Dakar, Senegal (S. Breurec).
From this initial group of H. pylori genomic DNA samples, 41 strains (Supplementary Table S2, strains 1 to 41) were selected for the presence of the target prophage genes, integrase and holin. These strains were then typed by MLST; for this purpose, seven housekeeping genes were amplified and sequenced, as previously described1.
The H. pylori genomes and WGS available at public databases were subjected to Blastn analysis19 for the presence of the integrase and holin prophage genes, with a threshold limit of <1e−6. The genomes carrying these genes were selected if isolated from human hosts. In the cases of multiple isolates from the same patient, only one genome was considered. In the selected genomes the two prophage genes and the seven MLST gene sequences were collected for further analysis along with the gene sequences of the 41 strains.
Implementation of a prophage sequence typing (PST) scheme for the prophages of H. pylori
Two prophage genes were selected to be used for the PST; integrase and holin. All strains were tested for the presence of integrase and positive strains were also tested for the presence of holin.
The collection of genomic DNA from H. pylori was first challenged by PCR for the presence of the integrase gene of the prophage, using the degenerated primers F1, AAGYTTTTTAGMGTTTTGYG and R1, CGCCCTGGCTTAGCATC (Eurofins Genomics, Ebersberg, Germany) which produce a 529-bp PCR product10. The strains carrying the integrase gene were then tested for the presence of holin. Degenerated primers based on the holin gene sequences of phages 1961P, KHP30, KHP40 and H. pylori strains Cuz20, India7 and H. acynonychis were used. The primers were hol-F CCATCCCGTATTTGTTGGTG and hol-R ACCCAATGCCTCCACTAATC (Eurofins Genomics) producing a 225-bp PCR product. In both cases, the PCR mix included Promega (Madison, Wisconsin, USA) buffer (1X), dNTPs (0.2 μM), primers (0.5 μM each), GoTaq polymerase (1.5 U), water to complete 25 μl and DNA sample (25 to 50 ng). The PCR cycle was composed of a first cycle at 95 °C for 4 minutes, 35 cycles at 95 °C for 30 seconds, 58 °C for 30 seconds and 72 °C for 1 minute. A last cycle at 72 °C for 7 minutes was applied.
The PCR products of each positive strain were purified using MicroSpin S-400 or S-300HR columns (GE Healthcare, Saclay, France) and directly sequenced on both strands using The BigDye® Terminator v3.1 Cycle Sequencing Kits (Applied Biosystems, Villebon-sur-Yvette, France), using an ABI 3700 analyzer DNA sequencer (PE Applied Biosystems).
To validate the reproducibility of this approach to identify prophages, a software for prophage detection17 in 53 complete H. pylori genomes (listed at REBASE genomes18 and retrieved from the NCBI in October 2014) was used.
H. pylori MLST and PST data analysis
The Structure 2.3.4.21,23,29 program was used to study the number of populations K using the admixture model for the H. pylori MLST and for PST. All gene sequences were first aligned and the file was converted to the STRUCTURE input file using xmfa2structure by X. Didelot and D. Falush (http://www.xavierdidelot.xtreemhost.com/clonalframe.htm). All runs were performed in duplicate. In each run, a Markov Chain Monte Carlo (MCMC) of 10,000 iterations and a burn-in period of 10,000 iterations were chosen. The highest mean value of ln likelihood was compared for multiple runs of 2 ≤ K ≤ 9 to select the K value that fit the best. For the H. pylori MLST analysis we included 741 other MLST profiles available on the PubMLST website (http://pubmlst.org/helicobacter/) taking into consideration previously used profiles1,2. The Structure 2.3.4. was also used, under the same conditions, for the analysis of the 41 strains plus 22 genomes (identified in public databases) after H. pylori MLST alone or with PST.
The trimmed and concatenated sequences of the seven H. pylori MLST genes from 741 strains available at PubMLST plus 41 strains included in this study and 22 genomes from public databases were aligned using MAFFT version730 and were used for the construction of a phylogenetic tree. A neighbour-joining phylogenetic tree topology of nucleotide alignments was constructed using the MEGA (Molecular Evolutionary Genetics Analysis) 6.0 software31, on the basis of distances estimated using the Kimura two-parameter model32. Branching significance was estimated using bootstrap confidence levels by randomly resampling the data 1,000 times with the referred evolutionary distance model. A phylogenetic tree was also constructed using only the sequences of the 41 strains included in the present work plus the sequences of the 22 genomes available in public databases. A similar approach was used for the construction of a phylogenetic tree of the trimmed and concatenated prophage genes, after alignment and elimination of poorly aligned positions and divergent regions of the alignment of DNA sequences using Gblocks33. These positions may not be homologous or may have been saturated by multiple substitutions and it is convenient to eliminate them prior to phylogenetic analysis.
Additional Information
Accession codes: The DNA sequences of the seven MLST genes are available at the PubMLST website (http://pubmlst.org/helicobacter/) with the profile numbers 2851 to 2886. The prophage sequences employed in the present study are available at GenBank (No: KM275873 to KM275935).
How to cite this article: Vale, F. F. et al. Dormant phages of Helicobacter pylori reveal distinct populations in Europe. Sci. Rep. 5, 14333; doi: 10.1038/srep14333 (2015).
References
Falush, D. et al. Traces of human migrations in Helicobacter pylori populations. Science 299, 1582–1585 (2003).
Linz, B. et al. An African origin for the intimate association between humans and Helicobacter pylori. Nature 445, 915–918 (2007).
Megraud, F. H pylori antibiotic resistance: prevalence, importance and advances in testing. Gut 53, 1374–1384 (2004).
Suerbaum, S. & Achtman, M. Helicobacter pylori: recombination, population structure and human migrations. Int J Med. Microbiol 294, 133–139 (2004).
Suzuki, R., Shiota, S. & Yamaoka, Y. Molecular epidemiology, population genetics and pathogenic role of Helicobacter pylori. Infect. Genet. Evol 12, 203–213 (2012).
Moodley, Y. et al. The peopling of the Pacific from a bacterial perspective. Science 323, 527–530 (2009).
Brussow, H., Canchaya, C. & Hardt, W. D. Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion. Microbiol. Mol. Biol. Rev. 68, 560–602 (2004).
Minot, S. et al. Rapid evolution of the human gut virome. Proc. Natl. Acad. Sci. U.S.A 110, 12450–12455 (2013).
Thiberge, J. M. et al. From array-based hybridization of Helicobacter pylori isolates to the complete genome sequence of an isolate associated with MALT lymphoma. BMC. Genomics 11, 368 (2010).
Lehours, P. et al. Genome sequencing reveals a phage in Helicobacter pylori. MBio. 2, e00239–11 (2011), 10.1128/mBio.00239-11.
Luo, C. H., Chiou, P. Y., Yang, C. Y. & Lin, N. T. Genome, integration and transduction of a novel temperate phage of Helicobacter pylori. J. Virol. 86, 8781–8792 (2012).
Uchiyama, J. et al. Complete Genome Sequences of Two Helicobacter pylori Bacteriophages Isolated from Japanese Patients. J. Virol. 86, 11400–11401 (2012).
Eppinger, M. et al. Who ate whom? Adaptive Helicobacter genomic changes that accompanied a host jump from early humans to large felines. PLoS. Genet. 2, e120 (2006).
Arnold, I. C. et al. Comparative whole genome sequence analysis of the carcinogenic bacterial model pathogen Helicobacter felis. Genome Biol. Evol. 3, 302–308 (2011).
Schott, T., Kondadi, P. K., Hanninen, M. L. & Rossi, M. Comparative genomics of Helicobacter pylori and the human-derived Helicobacter bizzozeronii CIII-1 strain reveal the molecular basis of the zoonotic nature of non-pylori gastric Helicobacter infections in humans. BMC. Genomics 12, 534 (2011).
Kersulyte, D., Rossi, M. & Berg, D. E. Sequence divergence and conservation in genomes of Helicobacter cetorum strains from a dolphin and a whale. PLoS. ONE. 8, e83177 (2013).
Zhou, Y., Liang, Y., Lynch, K. H., Dennis, J. J. & Wishart, D. S. PHAST: a fast phage search tool. Nucleic Acids Res 39, W347–W352 (2011).
Roberts, R. J., Vincze, T., Posfai, J. & Macelis, D. REBASE–a database for DNA restriction and modification: enzymes, genes and genomes. Nucleic Acids Res. 38, D234–D236 (2010).
Altschul, S. F. et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 (1997).
Yamaoka, Y. Helicobacter pylori typing as a tool for tracking human migration. Clin. Microbiol. Infect. 15, 829–834 (2009).
Pritchard, J. K., Stephens, M. & Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 155, 945–959 (2000).
Falush, D., Stephens, M. & Pritchard, J. K. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164, 1567–1587 (2003).
Falush, D., Stephens, M. & Pritchard, J. K. Inference of population structure using multilocus genotype data: dominant markers and null alleles. Mol. Ecol. Notes 7, 574–578 (2007).
Moodley, Y. et al. Age of the association between Helicobacter pylori and man. PLoS. Pathog. 8, e1002693 (2012).
Elhaik, E. et al. Geographic population structure analysis of worldwide human populations infers their biogeographical origins. Nat. Commun. 5, 3513 (2014).
Seldin, M. F. et al. European population substructure: clustering of northern and southern populations. PLoS. Genet. 2, e143 (2006).
Lazaridis, I. et al. Ancient human genomes suggest three ancestral populations for present-day Europeans. Nature 513, 409–413 (2014).
Elhaik, E. et al. Geographic population structure analysis of worldwide human populations infers their biogeographical origins. Nat. Commun. 5, 3513 (2014).
Falush, D., Stephens, M. & Pritchard, J. K. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164, 1567–1587 (2003).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol Evol. 30, 772–780 (2013).
Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol Evol. 30, 2725–2729 (2013).
Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16, 111–120 (1980).
Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol Evol. 17, 540–552 (2000).
Acknowledgements
This work was supported by the University of Malaya-Ministry of Education (UM-MoE) High Impact Research (HIR) Grant UM.C/HIR/MOHE/13/5 (h-50001-00-A000033) and by the Fundação para a Ciência e a Tecnologia (FCT) project grant PTDC/EBB-EBI/119860/2010. F.F.V. is recipient of a postdoctoral fellowship from FCT (SFRH/BPD/95125/2013). We are grateful to Xavier Didelot and Noah A. Rosenberg for technical support.
Author information
Authors and Affiliations
Contributions
F.F.V., P.L., J.V. and F.M. contributed to the design of the project. F.F.V., P.L., M.O., S.B., L.E. and T.T.P. selected the strains carrying prophages. F.F.V. analyzed the data and wrote the paper. All authors contributed to the paper and approved the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Vale, F., Vadivelu, J., Oleastro, M. et al. Dormant phages of Helicobacter pylori reveal distinct populations in Europe. Sci Rep 5, 14333 (2015). https://doi.org/10.1038/srep14333
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep14333
This article is cited by
-
Helicobacter pylori infection causes both protective and deleterious effects in human health and disease
Genes & Immunity (2021)
-
Comparative study between Helicobacter pylori and host human genetics in the Dominican Republic
BMC Evolutionary Biology (2019)
-
Tipping the Scale Towards Gastric Disease: a Host-Pathogen Genomic Mismatch?
Current Genetic Medicine Reports (2018)
-
Genomic structure and insertion sites of Helicobacter pylori prophages from various geographical origins
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.