Abstract
Genome-wide association studies have identified loci at 15q25 (IREB2) and 4q22 (FAM13A), associated with lung cancer (LC) and chronic obstructive pulmonary disease (COPD). The aim of our research was to determine the association of IREB2 and FAM13A SNPs with LC and severe/very severe COPD patients. We examined IREB2 variants (rs2568494, rs2656069, rs10851906, rs13180) and FAM13A (rs1903003, rs7671167, rs2869967) among 1.141 participants (468 LC, 149 COPD, 524 smoking controls). The frequency of the minor IREB2 rs2568494 AA genotype, was higher in LC vs controls (P = 0.0081, OR = 1.682). The FAM13A rs2869967 was associated with COPD (minor CC genotype: P = 0.0007, OR = 2.414). The rs1903003, rs7671167 FAM13A variants confer a protective effect on COPD (both P < 0.002, OR < 0.405). Haplotype-based tests identified an association of the IREB2 AAAT haplotype with LC (P = 0.0021, OR = 1.513) and FAM13A TTC with COPD (P = 0.0013, OR = 1.822). Cumulative genetic risk score analyses (CGRS), derived by adding risk alleles, revealed that the risk for COPD increased with the growing number of the FAM13A risk alleles. OR (95% CI) for carriers of ≥5 risk alleles reached 2.998 (1.8 to 4.97) compared to the controls. This study confirms that the IREB2 variants contribute to an increased risk of LC, whereas FAM13A predisposes to increased susceptibility to COPD.
Similar content being viewed by others
Introduction
Chronic obstructive pulmonary disease (COPD) and lung cancer (LC) are caused by the interaction between genetic susceptibility and environmental influences. Both diseases are leading causes of mortality and morbidity worldwide. In Poland, COPD is responsible for approximately 15.000 patients’ deaths per year and the clinical features of COPD are observed in about 10% of the Polish population, over 40 years of age1. Patients with COPD appear to be at increased risk of developing lung cancer, suggesting that there is a common mechanism which induces both diseases2. In addition, it was reported that the presence of mild emphysema, even without demonstrable obstruction, independent of cigarette-smoking burden, confers a substantial risk of lung cancer2. Although cigarette smoking is the main environmental risk factor, only about 10–20% of smokers develop COPD2 and only 10 to 15% of lung cancer cases arise in individuals who have never smoked3. Familial aggregation of both diseases has been observed. The connection between family history of LC and COPD was reported in a segregation analysis, in relatives of non-smoking cases4. Twin studies have also demonstrated that pulmonary function is heritable5. These data suggest a common underlying genetic determinant of susceptibility to lung diseases.
Several genome-wide association studies (GWASs) have identified genomic regions associated with COPD, LC and impaired lung function. These GWAS loci are located at IREB2 (iron-responsive element binding protein 2, MIM 613299) gene on chromosome 15q24-25.16,7,8,9 and in the 4q22.1 locus, including FAM13A (family with sequence similarity 13, member A, MIM 147582) gene10,11,12.
The IREB2 gene region (15q24) contains a number of genes encoding nicotinic acetylcholine receptor subunits (CHRNA3/CHRNA5/CHRNAB4 cluster for instance), which show strong association with COPD. This region was equally associated with LC6 and nicotine addiction13. The genes CHRNA3/CHRNA5 has been associated with the risk for nicotine dependence through mRNA brain expression levels14, dense genotyping15 and GWAs16 in various populations, including smokers with normal lung function and lung diseases17, like lung cancer and COPD. The identification of 15q24 region as a susceptibility loci in lung diseases, may suggest that LC and COPD are closely related and should not be considered separately, with particular emphasis on overlapping genetic effects18. Additionally, with the use of candidate gene approach, loci on 4q24 (INTS12), 6p21 (AGER) and 5p15 (ADCY2) have demonstrated the association with COPD19. An alternative approach to assess the cumulative effect of GWAs susceptibility in lung cancer after sub-phenotyping of COPD were submitted by Young R. et al. They have demonstrated that the risk genotypes from previously reported GWAs loci (including FAM13A and CHRNA3/5), incorporated into the algorithm with clinical variables, may estimate an overall genetic risk score20.
The IREB2 gene belongs to a group of genes which regulate mammalian iron homeostasis. IREB2 registers cytosolic iron status mainly through an iron-sulfur switch mechanism21. Considering its role as mediator of iron homeostastis and observed phenotype of IREB2 knock-out models, IREB2 gene is a candidate that could have an impact on many diseases’ pathogenesis. Coon et al. (2006) examined IREB2 gene polymorphisms in Alzheimer’s disease patients and demonstrated that two single nucleotide polymorphisms (SNPs) (rs2656070 and rs13180) were significantly associated with this disorder22. DeMeo et al. (2009) identified genomic regions from 56 lung tissue gene expression microarrays and used them to select SNPs, of which three IREB2 variants were associated with Norwegian COPD patients. In addition, the same investigators have observed increased levels of IREB2 mRNA and protein in lung tissue samples from COPD cases compared to healthy controls, which allowed them to propose IREB2 as a novel COPD susceptibility gene7.
The biological function of the FAM13A gene product is poorly understood. The highest expression of FAM13A gene was detected in the brain and ovaries, followed by the lungs and kidneys23. This gene encodes a protein with two coiled-coil domains and three nuclear localization signals24. It is suggested that the most important part of the FAM13A protein is its N-terminal extension containing the Rho-GAP domain, which presents tumour suppressor activity through inhibition of the intracellular signal transduction molecule RhoA18. It was shown that genetic variants in FAM13A gene may determine susceptibility to COPD and lung cancer18. The Rho GTPases activity regulation may indicated the potential role of FAM13A in carcinogenesis. Several such modulators of these type in immune cell migration and inflammation have been developed for cancers25. Recently, Z. Jin, J. Chung et al. have shown that knockdown of FAM13A significantly reduces the Wnt signaling activity in A549 human lung cancer cells26. Rho GTPases are also involved in the pulmonary endothelial barrier function in the lungs27, which is often dysregulated in lung diseases, such COPD and asthma28.
The current study aimed to determine the association of previously reported IREB2 and FAM13A SNPs with lung cancer and chronic obstructive pulmonary disease among two selected groups of Polish patients and smoking controls. The results of this study may elucidate whether genetic predisposition to COPD is shared with that of LC and suggest a new pathway in lung disease development.
Results
Genotype and allele distribution among patients and controls
The genotype/allele frequencies are summarized in Tables 1, 2, 3, 2, 2, 2, 3. The observed genotype frequencies of these 7 polymorphisms were all in agreement with the Hardy-Weinberg equilibrium (HWE) in the control subjects (for IREB2 HWE, the p-values were as follows: 0.674, 0.167, 0.149, 0.794; for FAM13A HWE p-values were: 0.986, 0.844, 0.766). We found that the IREB2 variants demonstrated an association with LC cases, while the FAM13A SNPs were associated with COPD.
For the IREB2 rs2568494 polymorphism, the AA genotype frequency, compared with the proportion of the GG genotype, was significantly higher in LC cases (P = 0.0081) than in the controls. Similarly, the presence of GA+AA genotypes (P = 0.0129) and the A allele (P = 0.0043) were more common among LC patients as compared to controls in single polymorphism analyses (Table 1). No association of IREB2 rs2568494 genotypes with the results of the pulmonary function tests: FEV1 and FEV1/VC (FEV1- forced expiratory volume in 1 second; VC- vital capacity; FEV1/VC- ratio) and smoking exposure in LC cases (Table 4) was noticed. We observed that frequencies of the G allele and GG genotype at rs2568494, G allele and GG genotype at rs10851906 and CC genotypes at rs13180 SNPs, were higher in controls compared to LC cases (Tables 1 and 3). The occurrence of the GG genotype of rs10851906 was differed greatly between LC cases and controls (P = 0.0027). When the IREB2 association was examined in the lung cancer cases without COPD (N = 369) compared to controls the results were comparable to those received for general lung cancer group (N = 468). In case of rs2568494 SNP the association was found but was not significant after Bonferroni correction (AA genotype: P = 0.0206, OR = 1.62 [1.08–2.44] and GA+GG: P = 0.0457, OR = 1.32 [1–1.73]; A allele: P = 0.0154, OR = 1.27 [1.05–1.55]). The occurrence of rs10851906 GG genotype was different between LC no-COPD cases and controls (P = 0.0087, OR = 0.4 [0.2–0.81]). The frequencies of CC genotype and C allele at rs13180, were higher in controls compared to LC no-COPD cases (CC genotype: P = 0.0098, OR = 0.57 [0.37–0.88]; CT+CC genotype: P = 0.015, OR = 0.71 [0.55–0.94] and C allele: P = 0.0052, OR = 0.75 [0.62–0.92]).
The interactions of selected genotypes of IREB2 (rs2568494, rs10851906, rs13180) with age that influenced the LC risk were observed (Fig. 1). For three IREB2 SNPs the results obtained for genotypes interacted with age were: rs2568494 GG OR = 5.7 95%CI [3.7–8.9], GA OR = 5.6 95%CI [3.7–8.4], AA OR = 8.3 95%CI [3.7–18]; rs10851906 AA OR = 5.6 95%CI [3.0–8.1], AG OR = 5.5 95%CI [3.4–8.9], GG OR = 19.3 95%CI [3.9–97] and rs13180 TT OR = 5.7 95%CI [3.7–8.8], CT OR = 6 95%CI [4–9.2], CC OR = 6.8 95%CI [3.1–15]. No significant associations were found in case of sex variable (P > 0.05) (Fig. 1).
Significant influences of IREB2 and FAM13A SNPs on COPD and lung cancer according to age and sex.
A multiple linear regression analysis was performed for IREB2 (rs2568494, rs10851906, rs13180) genotypes in LC and FAM13A (rs7671167, rs1903003, rs2869967) genotypes in COPD. The odds ratios with 95% confidence intervals (O.R. with 95%CI) for genotypes of selected SNPs are displayed. Control group was set as reference group. All results for age variable were statistically significant. LC: lung cancer; COPD: chronic obstructive pulmonary disease.
Each of the three FAM13A SNPs showed a significant difference in genotype/allele frequency between COPD cases and controls (Tables 2, 3). The rs2869967 CC genotype was closely associated with COPD (P = 0.0007), also when the COPD with LC+COPD patients was analyzed (combined COPD with LC+COPD; P < 0.0001). Similarly, the frequency of the C allele was significantly higher in these two groups (P = 0.0009 and 0.0001, respectively) compared with the controls. We found no association of FAM13A rs2869967 genotypes with lung function measurements FEV1 and FEV1/FVC (FVC- forced vital capacity; FEV1/FVC - ratio) and smoking exposure in the COPD (Table 4). The TC, CC and TC+CC genotypes of rs7671167 SNP were more common in the control group as compared to COPD (P = 0.022, < 0.0001, 0.0015) and COPD with LC+COPD groups (P = 0.036, 0.0004, 0.003) (Table 2). These results were consistent with the difference in C allele frequency between these two groups of patients (P = 0.0002, 0.0003) (Table 3). Likewise, in case of rs1903003 SNP, the presence of the CT, CC and CT+CC genotypes was significantly more frequent among the controls compared to COPD (P = 0.024, 0.0014, 0.0028) and COPD with LC+COPD groups (P = 0.028, 0.0002, 0.0019) (Table 2). These observations were compatible with C allele distribution in these two analyzed groups of COPD patients analyzed (P = 0.0009, 0.0002) (Table 3). The interactions of FAM13A (rs1903003, rs7671167, rs2869967) genotypes with age that influenced the COPD risk were detected (Fig. 1). For each FAM13 SNPs the results obtained for genotypes interacted with age were: rs7671167 TT OR = 3.3 95%CI [1.6–6.6], TC OR = 9.5 95%CI [14.8–18], CC OR = 26 95%CI [6.5–105]; rs1903003 TT OR = 4 95%CI [2–7.7], CT OR = 8.4 95%CI [8.4–16], CC OR = 36 95%CI [7–136] and rs2869967 TT OR = 25 95%CI [8–81.6], CT OR = 7 95%CI [3.6–13], CC OR = 3.3 95%CI [1.4–7.5]. No significant associations were found in the case of the sex variable (P > 0.05). None of the FAM13A SNPs showed significant associations with lung cancer cases (Fig. 1).
Multiple testing of SNP-SNP interactions
Logistic regression was used to adjust the effects of studied SNPs in impact in occurrence of LC (4 SNPs in IREB2 gene), COPD and combined COPD with LC+COPD (3 SNPs in FAM13A gene). Each analysis including all studied SNPs from particular gene as covariate. In LC, IREB2 rs252568494 revealed the strongest association (P = 0.037; OR = 1.2; 95%CI: 1–1.5) after correction of influence of the three other SNPs. In COPD, FAM13A rs7671167 was the most significantly associated (P = 0.029; OR = 0.7; 95%CI: 0.5–0.97), whereas in combined COPD with LC+COPD, the FAM13A rs2869967 was the most relevant (P = 0.04; OR = 1.3; 95%CI: 1–1.7).
Linkage disequilibrium analysis
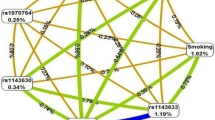
Linkage disequilibrium (LD) calculations (Levontin’s D’ value and correlation coefficient r2) were done for each pair of the 4 investigated IREB2 polymorphisms and 3 SNPs of FAM13A gene. The values for LD between SNPs are shown in Fig. 2. The pairwise LD values suggested strong linkage between studied SNPs in both genes. Within IREB2 gene there is strong LD between rs2656069 and rs10851906 (r2 = 0.95). Within FAM13A, SNPs rs7671167 and rs1903003 are in LD (r2 = 0.84).
Frequency of IREB2 and FAM13A haplotypes
To analyze the combined effect of these four investigated IREB2 SNPs and 3 SNPs of FAM13, we the generated the haplotypes on the basis of the genotyping data observed. The construction of haplotypes revealed the presence of seven IREB2 haplotypes and 6 FAM13A haplotypes in COPD/LC patients and controls. Haplotypes with a frequency lower than 1% in all groups were not considered for further analysis. Finally, we analyzed the eight most common haplotypes (four for each gene; Table 5). The most frequent haplotypes in patients and controls were: AAAT (36%), GAAT (27%), GGGC (21%), GAAC (14%) for IREB2 and CCT (43%), TTC (41%), TTT (10%), CTT (3%) for FAM13A. The IREB2 AAAT haplotype was associated with an increased risk of LC (P = 0.0021; OR = 1.51; 95%CI: 1.62–1.97). In the case of FAM13A gene, the frequencies of haplotype TTC were significantly higher in COPD patients and COPD combined with LC+COPD group than in the controls (P = 0.0013; OR = 1.82; 95%CI: 1.26–2.63; and P = 0.0003; OR = 1.76; 95%CI: 1.29–2.39, respectively).
Cumulative genetic risk score
All study variants in both genes indicated a significant association with LC or COPD were included in the cumulative genetic risk score analysis (CGRS). The CGRS of two IREB2 SNPs (rs2568494, rs13180) among LC did not a reveal significant increase in OR value: for carriers of ≥3 risk alleles OR reached 1.5957 (1.1717 to 2.1730). In FAM13A gene the risk alleles were defined as T for rs13180, rs7671167 and A for rs2568494. The average (±SD) of cumulative risk scores among COPD patients (3.54 ± 1.97) and mixed COPD with LC+COPD group (3.459 ± 1.96) were significantly higher than the controls (2.857 ± 1.83) with a P value < 0.0001. We also observed a significant difference in the average (±SD) of weighted cumulative risk scores comparing COPD and COPD with LC+COPD to the control group (3.54 ± 1.98 and 3.46 ± 1.96 vs. 2.86 ± 1.83, respectively, P < 0.0001). Individuals were stratified according to the number of risk alleles into group carrying ≤2 (reference group), 3, 4 and ≥5 alleles. The risk of COPD increased with the growing number of the FAM13A risk alleles. OR (95% CI) for COPD carriers of ≥5 risk alleles reached 2.998 (1.8092 to 4.9678) for unweighted CGRS (Fig. 3). In COPD with LC+COPD group, odds ratio for carriers of ≥5 risk alleles amounted to 2.542 (1.6705 to 3.8693) for unweighted CGRS (Fig. 3). The odds ratios calculated in unweighted and weighted CGRS analysis were similar, which is probably connected with the low number of risk alleles and small sample size.
Cumulative genetic risk score (CGRS) analysis of FAM13A SNPs.
The effect of unweighted cumulative genetic risk score on COPD and COPD with LC+COPD was calculated using logistic regression analysis. The odds ratios (black symbols) with 95% confidence intervals (range bars) for the number of risk alleles at each of FAM13A SNPs are represented from unweigheted analysis. COPD: chronic obstructive pulmonary disease; LC: lung cancer; COPD with LC+COPD: COPD with 40% admixture of subjects with concomitant LC.
Statistical power analysis
The post-hoc analysis revealed that the statistical power of our study for analyses of the differences in genotype distribution between subgroups of patients and controls ranged between 80–97%. The statistical power of the study to detect the IREB2 alleles associated with LC (OR: 0.7 to 1.4) for the three SNPs with a frequency of 0.2 to 0.38 (in the additive model) was 80–97%. The analysis of rs10851906 alleles in LC group, in the recessive model, revealed that the power of study is 80%. In case of FAM13A alleles associated with COPD and combined COPD with LC+COPD group (OR: 0.5 to 1.7) with a frequency of 0.3 to 0.5 (in the additive model) the statistical power was 91–97%. This means that the study had sufficient power to detect an association of the IREB2 gene in LC and FAM13A gene in COPD and combined COPD with LC+COPD group, in the case-control analysis.
Discussion
In this research we examined 7 SNPs among patients with LC, COPD and in healthy controls from Poland (n = 1141), in an attempt to evaluate the association of IREB2 and FAM13A loci with these diseases. In our study we observed that IREB2 variants appeared to be associated with LC, whereas the FAM13A was linked with COPD.
The IREB2 variants have been previously linked to COPD as well as lung cancer. Three independent groups performing GWA studies have identified 15q25 locus, comprising the IREB2 gene, as associated with lung cancer6,8,9. In our study, three out of four selected SNPs showed a significant association with the LC, whereas none of them reached the level of significance among COPD cases (Table 2). The genotype AA of IREB2 rs2568494 was significantly more frequent among LC group (P = 0.0081; OR = 1.682), which suggested that this variant might increase the risk of lung cancer. This association was independent of lung function among cases (FEV1 and FEV1/VC) and smoking intensity (Table 6). However, the effect of the abovementioned variant and age of lung cancer development was observed (Fig. 1). The prevalence of rs10851906 GG and rs13180 CC genotypes was significantly higher among the controls compared with LC cases, which suggests that minor alleles of these SNPs may have an apparently protective effect (OR = 0.379, 0.595, respectively). Our results are difficult to interpret because of the low number of reports on the protective effect of single IREB2 gene polymorphisms. Among 4 IREB2 haplotypes, only one was associated with an increased risk of LC. In the current study, the IREB2 locus was also examined in the lung cancer cases without COPD and the results just reflect the overall case-control relationship. However, it is worth noting that the lung cancer patients selected for this study, were only those considered for surgery. This may explain the low rate of COPD patients in this group (21%), whereas the prevalence of COPD in several lung cancer cases is reported to between 50–70%18,29. An alternative approach to estimate an overall genetic risk score, by the multivariate analysis that include genotypes and clinical variables which are independent predictors of lung cancer, were proposed by R. Young et al. In this report, the SNPs data were used to compile the risk model, which includes many risk genotypes from genes implicated in both COPD and lung cancer. These multifactorial approach may be helpful in identifying genes underlying the development of lung cancer20.
In contrast to COPD, the potential role of the IREB2 gene in human cancer is not well documented. Recently, studies on mouse models showed that overexpression of IREB2 promoted the growth of tumor xenografts in nude mice. In addition, the authors have noticed that the unique conserved insert of 73 amino acids of IREB2 mammalian protein, contributes to IREB2 pro-oncogenic potential and is essential for accelerated tumor growth30. However, a quantitative analysis of IREB2 mRNA levels in paired normal lung and lung adenocarcinoma tissue demonstrated the increased expression of CHRNA5, but no change in IREB231. In another study, using gene overexpression and/or gene knockdown and apoptosis analyses, the authors have failed to reveal that IREB2 mediates effects on lung cancer cell growth in vitro32. Further studies are needed to prove the link between IREB2 protein and cancer biology.
DeMeo et al. (2009) have demonstrated that IREB2 may be a COPD susceptibility gene, identified through the integration of lung microarray expression data and replicated genetic association in many Caucasian cohorts7,33,34, including the Polish population35. Nevertheless, we were not able to confirm these findings in our group of Polish patients with COPD. IREB2 is located in the region near the CHRNA3/CHRNA5/CHRNAB4 genes cluster with a possible role of lung diseases development. The significant LD in this region a is source of difficulties in association analyses. M. Hardin et al. (2012) have demonstrated a significant association of IREB2 rs13180 with 315 severe COPD cases from Poland (P = 3.4 × 10−3; OR = 0.69)35. However, after adjustment for the presence of the CHRNA3/5 SNP, this association was no longer significant, indicating that the positive results may be driven by LD with CHRNA3/5 polymorphism.
The functional consequences of IREB2 polymorphism are unclear. The protein product of the IREB2 gene is involved in maintaining human cellular iron metabolism. It has been demonstrated that smokers had higher concentrations of iron in the lungs36. Iron imbalance may lead to oxidative injury, whereas an excess of iron has an impact upon regional inflammation in the lungs, which may be relevant to the pathogenesis of COPD and lung cancer. It is supposed that IREB2 variants may further affect COPD and lung cancer when coupled with the increased levels of iron accumulated through exposure to cigarette smoke7.
We confirmed previous observations, which suggest that FAM13A variants may play a role in COPD susceptibility for all three SNPs selected for this study. The statistically significant values were obtained in almost each genotype with a minor allele in COPD patients and in the COPD group merged with patients suffering from concomitant LC and COPD (Table 3). It is not surprising, that the results for all SNPs were similar, due to the high level of linkage disequilibrium between these three SNP (CEU HapMap, NCBI B36 assembly)18. Among three FAM13A variants, only rs2869967 showed an association with an increased risk of COPD, whereas rs7671167 and rs1903003 were significantly more frequent among the controls (Table 3). A positive effect of these SNPs on age at COPD occurrence was observed (Fig. 1).
The rs2869967 CC genotype was significantly more frequent among the COPD group (P = 0.0007; OR = 2.414) and in the COPD group combined with LC+COPD (P < 0.0001; OR = 2.358). This variant has been mainly investigated in large Chinese populations and its CC genotype frequency seemed to be increased37,38. Wang et al. showed that rs7671167 and rs2869967 were associated with the FEV1/FVC ratio among all subjects, whereas rs2869967 revealed only a borderline association (P = 0.05) with COPD cases after adjusting for age, gender, body mass index (BMI), pack-years of smoking and current smoking status38. Further investigations including functional analyses are warranted to confirm the impact of CC rs2869967 variant in the modulation of the COPD risk.
The prevalence of CC genotypes of rs7671167 and rs1903003 was significantly elevated among the controls compared with COPD cases, which suggests the protective effect of these alleles (Table 3). Here, we confirm the findings of M. Cho et al. among non-Hispanic white, African-American and Norwegian cases, followed by R. Young et al. in Caucasians. In the first study, conducted among 2,940 COPD cases and 1,380 controls with normal lung function, the locus most highly associated with COPD included two SNPs: rs1903003 and rs7671167 in high linkage disequilibrium (r2 = 0.85). Subsequently, they genotyped these two SNPs in 502 COPD cases and 504 controls and in two large family-based cohorts including 3,808 cases and confirmed the associations. Similarly, the study by R. Young et al. demonstrated that the C allele of rs7671167 is associated with a reduced risk of COPD18. In addition, they have indicated its protective effect in COPD combined with lung cancer patients with pre-existing COPD, which is in agreement with our observations in COPD combined with LC+COPD. However, these authors also showed, that this variant was associated with reduced risk of lung cancer (OR = 0.75, P = 0.002). This relationship was absent in our study, because no significant difference was detected in the frequencies of genotypes/alleles of FAM13A among lung cancer patients. Another study35, carried out among 305 Polish patients with severe and very severe COPD, did not demonstrate a significant association between the rs7671167 SNP and COPD. However, they indicated that the C allele was associated with improved lung function, both in COPD cases and control population35. The association of FAM13A variants with lung function was replicated in several studies11,12. D. Hancock et al. meta-analyzed GWA studies, including 20.890 participants11. A phenotype, described by two important lung-function parameters: FEV1 and FEV1/FVC, is inheritable and provides the basis for evaluation of lung function. Hancock et al. identified eight loci associated with FEV1/FVC, including also variants in the FAM13A gene, namely rs2869967, which was analysed in our study. In current study, we did not identify an association between this SNP and FEV1 and FEV1/FVC parameters, as well as smoking intensity (Table 6). Among the 4 FAM13A haplotypes selected for this study, the prevalence of TTC was significantly higher in COPD patients than in controls.
We also observed a cumulative effect of three SNPs in the FAM13A locus. The risk of COPD escalated with an increasing number of risk alleles. Although the tendency for increasing OR for individuals with four risk alleles was moderate, probably due to the small number of carriers in this group, among carriers of ≥5 risk alleles OR rose significantly to 2.998. These results might suggest an additive effect of the variants being studied.
Whether any of these three SNPs is functional remains unknown, although given their location, an effect on splice variants would be possible. The most statistically significant result in our study was obtained for rs7671167 SNP, which lies in intron 4 of the FAM13A gene and was identified by Cho et al. It localizes downstream of the most important part of the FAM13A molecule: the Rho-GAP domain. Although little is known of the FAM13A function, the Rho GTPase activating role suggests both anti-inflammatory and tumor suppressor activity for FAM13A protein. FAM13A gene expression analyses in cell lines from several tissues (not comprising the lung) have shown a consistent increase in its expression in response to hypoxia39. Differences in the expression of this gene have also been noted in respiratory epithelial cells during differentiation into pulmonary type II alveolar cells in vitro model and among individuals with mild as compared to severe cystic fibrosis40. Recently, Z. Jin, J. Chung et al. showed that depletion of FAM13A in human lung cancer cells causes a reduction in Wnt signaling activity which provides evidence that Fam13a may contribute to human lung diseases26.
The effects of studied SNPs in impact in LC occurrence, COPD and combined COPD with LC+COPD were analysed using multiple testing of SNP-SNP interactions. In LC, IREB2 rs252568494 revealed the strongest association. In COPD, FAM13A rs7671167 was the most significantly associated, whereas in combined COPD with LC+COPD, the FAM13A rs2869967 was the most relevant. After adjustment of the influence of the other SNPs in each gene, these associations were still significant (P < 0.05). Despite the loss of significance after Bonferroni correction, the direction of SNP effect remained the same. It is possible that the strong LD between certain SNP may cause the decrease of P-value level. These SNPs may be independent predictors of phenotype in pulmonary diseases and should be considered in future studies concerning the development of gene-based prognostic scores for LC and COPD.
There are some limitations to the current study that need to be addressed. The number of COPD patients is limited, but the group was very accurately selected and the history of the disease and also exposition to noxious substances were collected in detail. 75% of cases had severe or very severe airflow limitation in the course of the disease (stage III and IV in GOLD classification), which represents more extreme phenotype of this disease. Although limited by our sample size, we will still able to demonstrate several significant associations in case of both genes in all groups of cases vs controls. With a Bonferroni correction for multiple testing, which is very conservative approach, the associations between IREB2 and lung cancer and FAM13A and COPD remain significant. Although, many data had provided evidence for a role of IREB2 gene in COPD, we were not able to demonstrated this associations. It is possible that association in IREB2 locus may be driven by LD with CHRNA3/5 SNPs only, but these variants were not examined in current study. It is also possible that IREB2 SNPs have not an impact in severe/very severe COPD occurrence. We did not demonstrated significant associations, however small effect was found in rs2568494 AA genotype in the combined COPD with LC+COPD group. Although the cohort of COPD cases were pooled restrictively, it seems to be underpowered to detect an effect at the IREB2 locus. However, the statistical power for our analyses ranged between 80–97%, which may suggest that the study had sufficient power to detect an association.
Another limitations of the current study, is connected with smoking controls selection. Controls were matched by sex with both groups of patients (~70% of males in all groups). However, in the case of age distribution considerable deviation was observed between COPD and lung cancer cases and controls (median age 66, 64 and 55 years, respectively), which may lead to bias being introduced. The smoking status of controls was not matched exactly, because information about smoking exposure measures (like cigarettes/day or pack-years) was not available for 40% of controls. In this subgroup, we were not able to calculate pack years, because no information about all smoking exposure measures were pooled.
The control group included only cancer-free cases with normal pulmonary function, which allowed us to simultaneously compare COPD and lung cancer groups with the matched controls. This study design enabled us to determine the possible link between COPD and lung cancer patients related to IREB2 and FAM13A variants. To our knowledge, this is the first report in which IREB2 and FAM13A genes were analyzed in parallel among COPD and lung cancer cases, with cumulative genetic risk score analyses. This approach provided an assessment of the potential role of IREB2 and FAM13A genes in lung disease development in the Polish population. To date, only R. Young et al. have examined one variant of the FAM13A gene in case-control studies of COPD, lung cancer and controls11.
Conclusion
We confirmed the association between IREB2 gene and lung cancer and between FAM13A gene and COPD in Polish patients. Further studies are required to elucidate the functional role of these variants, which may have an impact on lung disease susceptibility.
Methods
All experiments were performed in accordance with relevant guidelines and regulations approved by the Ethics Committee of the Poznan University of Medical Sciences (decision no. 802/10). All subjects gave their written informed consent to participate.
Materials
All subjects were of Caucasian ancestry, recruited from Wielkopolska region in Poland. The detailed characteristics of patients and controls are displayed in Table 6.
LC Patients
The cohort of 468 LC cases was recruited in the Wielkopolska Center of Pulmonology and Thoracosurgery from patients scheduled for surgical treatment. Most of them were males (69%), with median age of 63.5 years (range 51–78 years). The diagnosis of lung cancer was confirmed by the histopathological examination of the resected or biopsied tissue specimens in all cases. 97% of cases were non-small cell lung cancer (NSCLC). Spirometry was done in all LC patients and the median values of measurements were as follows: FEV1%: 89%, VC%: 99% and FEV1/VC: 94%. 91% patients had positive history of tobacco smoking.
COPD Patients
COPD subjects (N = 149, 72% males) were recruited in the Department of Pulmonology, Allergology and Respiratory Oncology, Poznan University of Medical Sciences. Median age of COPD patients was 66 years (range 39–87 years; of which 40 cases = <60 years). Inclusion criteria for stable COPD comprised airflow limitation as indicated by post-bronchodilator FEV1/FVC <0,7 and FEV1 ≤70% of normal predicted values. Patients with established diagnosis of asthma, LC, history of atopy, known AAT (α1-antitrypsin) deficiency or serum AAT level of less than 1.0 g/L were excluded from the study. Severity of airflow limitation was classified according to GOLD standard (Global Initiative for Chronic Obstructive Lung Disease) as follows: II—moderate, III—severe, IV—very severe. Most of them had positive history of tobacco smoking (97.7%). The mean value of pack-years of smoking was 43 ± 6.9.
COPD with LC+COPD Patients
In 99 (21%) out of 468 LC patients, COPD was diagnosed as the concomitant disease, which allowed us to distinguish the subgroup of LC patients with coexisting COPD (LC+COPD, N = 248). This subgroup was combined with COPD patients and analyzed separately as the COPD subjects with 40% admixture of subjects with concomitant LC (COPD with LC+COPD).
Controls
Control subjects (N = 524) demonstrated normal lung function with no evidence of airflow limitation (FEV1/FVC > 0.7). This group consisted of individuals for screening check-up in hospital or healthy blood donors with negative history of medical illnesses recruited at the Regional Blood Transfusion Centre. Efforts were undertaken to frequency-match cases by ethnicity, age and sex. Most of them were males (72%), with median age of 55 years (range 35–80 years). All controls were treated as smokers: current (29%), former (71%). Among former smokers were two groups: quitting smoking less than year (23%) and more than year (48%).
Methods
DNA samples from COPD patients, LC patients and controls were isolated from peripheral blood lymphocytes by Gentra Puregene Blood Kit (Qiagen, Hilden, Germany). DNA purity and concentration was confirmed using NanoDrop ND-1000 spectrophotometer.
We selected the SNPs previously associated with lung function and/or COPD in GWAs. We chose genomic regions based on review of the literature and used the most significant reported SNPs which were analyzed in relatively large groups of cases. For IREB2 gene we selected the SNPs described by D. De Meo et al.7, for FAM13A gene we chose the SNPs which were identified by M. Cho et al.10. All polymorphisms selected for this study had minor allele frequencies >0.4 in order to achieve sufficient statistical power. Altogether four SNPs in IREB2 (rs2568494, rs2656069, rs10851906, rs13180) and three variants of FAM13A (rs1903003, rs7671167, rs2869967) were analysed.
The SNPs were genotyped using pre-designed TaqMan® SNP genotyping assays (Life Technologies, Carlsbad, California; assays IDs: IREB2: 16043098_10, 15916464_10, 11522538_10, 8873396_1; FAM13A: 1143659_10, 1143656_10, 15837681_10)41. The PCR was performed with HOT FIREPol Probe qPCR Mix Plus (no ROX) according to the manufacturer’s instructions provided by Solis Biodyne (Tartu, Estonia). The PCR thermal cycling was as follows: initial denaturation at 95 °C for 15 min.; 40 cycles of 95 °C for 15 sec and 60 °C for 60 sec. Thermal cycling was performed using a CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad, Hercules, California, U.S.). As a quality control measure, negative controls and approximately 5% of samples were genotyped in duplicate to check genotyping accuracy. The genotypes of selected samples were confirmed by direct sequencing (OLIGO, IBB, Warszawa, Poland).
Statistical analysis
The Hardy–Weinberg equilibrium (p2 + 2pq + q2 = 1, where p is the frequency of the variant allele and q = 1−p) was tested by chi-square test to compare the observed and expected genotype frequencies in healthy controls. The statistical differences in genotype and allele frequencies between the studied groups and controls were also evaluated by the chi-square test. For all polymorphisms, the ancestral or major allele was considered wild-type and the homozygous wild-type genotype was considered the referent genotype. Odds ratios (ORs) and 95% confidence intervals (CIs) for association between the IREB2 or FAM13A genotypes and alleles and studied diseases were estimated using logistic regression analysis. Each patients group (COPD and LC) was examined as an independent case series. An extended COPD cohort, including COPD cases and LC patients with coexisting COPD (COPD with LC+COPD) was additionally considered in a separate case-control analysis. Statistical calculations were performed using GraphPad PRISM 5, module Statistical Analysis, Contingency Tables (GraphPad Software Inc.San Diego, CA).
Linkage disequilibrium (LD) measures were used to detect associations between the 4 investigated IREB2 polymorphisms and 3 SNPs of FAM13A gene. On the basis of the genotype data of all subjects, pair-wise LD was calculated using 2 standardized LD coefficients: r2 and Levontin’s D’. These associations were also tested for significance by means of the chi-square test. The expectation-maximization (EM) algorithm was applied to generate maximum likelihood estimates of haplotype frequencies. It was assumed that the Hardy–Weinberg equilibrium is applicable to the constructed haplotypes based on observed genotypes of IREB2 and FAM13A genes. Finally, the associations between specific haplotypes and the disease were studied among patients and controls (using chi-square test). To account for false-positive findings, multiple testing Bonferroni correction was applied. Because we tested 2 genes in 2 groups of patients, the result is significant if the corrected P-value is below the cutoff of <0.0125 [P-value * n (number of genes in test) <0.05]42. Calculations were performed using HAPLOVIEW, Linkage format [http://www.broad.mit.edu/mpg/haplo-view]. In case of two SNPs, which appeared significantly more frequent in patients’ groups in relation to controls, frequency of genotypes, lung function measures and smoking exposure were compared by One-way ANOVA, Ordinary test. Association between rs2568494 IREB2 variant and lung function measures (FEV1, FEV1/VC) was analyzed in the lung cancer cases, whereas rs2869967 FAM13A variant and COPD phenotype (FEV1, FEV1/FVC) was compared among COPD patients. Smoking exposure was set as a quantitative feature (pack-years of smoking). A multiple linear regression analysis was used to investigate the correlation of variables, including age, sex and genotype with disease by Logistic Regression 05.07.20 program (http://statpages.org/logistic.html). Effects of selected IREB2 and FAM13A genotypes on COPD/LC development, according to sex and age were estimated with the use of control group as a reference category. Logistic regression was used to multiple testing of SNP-SNP interactions by STATISTICA version 10.0 software. QUANTO software (version 1.2.4) was employed for sample size calculations.
Cumulative genetic risk score
SNPs in FAM13A showing significant association with COPD were included in the cumulative genetic risk score (CGRS) analysis. Genotypes for each SNP were coded as 0, 1 or 2 indicating the number of COPD risk alleles in the genotype. Unweighted and weighted CGRS (wCGRS) were calculated. In an unweighted approach the number of risk variants carried by an individual at each FAM13A SNP was counted to create cumulative genetic risk score (possible score for three SNPs range 0–6). In a weighted approach, OR estimated for risk allele in current study was used (T vs. C, OR 1.634; 1.565 and C vs. T, OR 1.548 of rs7671167, rs1903003 and rs2869967, respectively). A weighted risk score is the sum of the weighted COPD allele counts, weighted by natural logarithm of odds ratio for each risk allele ln(OR) and scaled by factor 3/w1 + w2 + w3 where wi = ln(OR) for the ith SNP and i = 1 to 343. Only samples without missing genotype were included in the analysis (COPD n = 149, Controls n = 524).
The effect of unweighted and weighted cumulative genetic risk score on COPD was calculated using logistic regression analysis. To compare the average (±SD) of CGRS and wCGRS between COPD patients and controls the unpaired t test was applied.
Additional Information
How to cite this article: Ziółkowska-Suchanek, I. et al. Susceptibility loci in lung cancer and COPD: association of IREB2 and FAM13A with pulmonary diseases. Sci. Rep. 5, 13502; doi: 10.1038/srep13502 (2015).
References
Makowska, M., Romanowicz, H., Kulig, A. & Smolarz, B. [Genetics risk factors in chronic obstructive pulmonary disease]. Polski merkuriusz lekarski: organ Polskiego Towarzystwa Lekarskiego 28, 302–306 (2010).
Houghton, A. M., Mouded, M. & Shapiro, S. D. Common origins of lung cancer and COPD. Nature medicine 14, 1023–1024, 10.1038/nm1008-1023 (2008).
Thun, M. J. et al. Lung cancer death rates in lifelong nonsmokers. Journal of the National Cancer Institute 98, 691–699, 10.1093/jnci/djj187 (2006).
Schwartz, A. G. & Ruckdeschel, J. C. Familial lung cancer: genetic susceptibility and relationship to chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine 173, 16–22, 10.1164/rccm.200502-235PP (2006).
Redline, S. et al. Genotypic and phenotypic similarities in pulmonary function among family members of adult monozygotic and dizygotic twins. American journal of epidemiology 129, 827–836 (1989).
Amos, C. I. et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nature genetics 40, 616–622, 10.1038/ng.109 (2008).
DeMeo, D. L. et al. Integration of genomic and genetic approaches implicates IREB2 as a COPD susceptibility gene. American journal of human genetics 85, 493–502, 10.1016/j.ajhg.2009.09.004 (2009).
Hung, R. J. et al. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature 452, 633–637, 10.1038/nature06885 (2008).
Thorgeirsson, T. E. et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature 452, 638–642, 10.1038/nature06846 (2008).
Cho, M. H. et al. Variants in FAM13A are associated with chronic obstructive pulmonary disease. Nature genetics 42, 200–202, 10.1038/ng.535 (2010).
Hancock, D. B. et al. Meta-analyses of genome-wide association studies identify multiple loci associated with pulmonary function. Nature genetics 42, 45–52, 10.1038/ng.500 (2010).
Pillai, S. G. et al. Loci identified by genome-wide association studies influence different disease-related phenotypes in chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine 182, 1498–1505, 10.1164/rccm.201002-0151OC (2010).
Spitz, M. R., Amos, C. I., Dong, Q., Lin, J. & Wu, X. The CHRNA5-A3 region on chromosome 15q24-25.1 is a risk factor both for nicotine dependence and for lung cancer. Journal of the National Cancer Institute 100, 1552–1556, 10.1093/jnci/djn363 (2008).
Wang, J. C. et al. Risk for nicotine dependence and lung cancer is conferred by mRNA expression levels and amino acid change in CHRNA5. Human molecular genetics 18, 3125–3135, 10.1093/hmg/ddp231 (2009).
Saccone, N. L. et al. The CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster affects risk for nicotine dependence in African-Americans and in European-Americans. Cancer research 69, 6848–6856, 10.1158/0008-5472.CAN-09-0786 (2009).
Pillai, S. G. et al. A genome-wide association study in chronic obstructive pulmonary disease (COPD): identification of two major susceptibility loci. PLoS genetics 5, e1000421, 10.1371/journal.pgen.1000421 (2009).
Saccone, N. L. et al. Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD. PLoS genetics 6, 10.1371/journal.pgen.1001053 (2010).
Young, R. P. et al. FAM13A locus in COPD is independently associated with lung cancer—evidence of a molecular genetic link between COPD and lung cancer. The application of clinical genetics 4, 1–10, 10.2147/TACG.S15758 (2011).
Castaldi, P. J. et al. The association of genome-wide significant spirometric loci with chronic obstructive pulmonary disease susceptibility. American journal of respiratory cell and molecular biology 45, 1147–1153, 10.1165/rcmb.2011-0055OC (2011).
Young, R. P. et al. Individual and cumulative effects of GWAS susceptibility loci in lung cancer: associations after sub-phenotyping for COPD. PloS one 6, e16476, 10.1371/journal.pone.0016476 (2011).
Rouault, T. A. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nature chemical biology 2, 406–414, 10.1038/nchembio807 (2006).
Coon, K. D. et al. Preliminary demonstration of an allelic association of the IREB2 gene with Alzheimer’s disease. Journal of Alzheimer’s disease: JAD 9, 225–233 (2006).
Nagase, T. et al. Prediction of the coding sequences of unidentified human genes. IX. The complete sequences of 100 new cDNA clones from brain which can code for large proteins in vitro. DNA research: an international journal for rapid publication of reports on genes and genomes 5, 31–39 (1998).
Cohen, M. et al. Cloning and characterization of FAM13A1—a gene near a milk protein QTL on BTA6: evidence for population-wide linkage disequilibrium in Israeli Holsteins. Genomics 84, 374–383, 10.1016/j.ygeno.2004.03.005 (2004).
Biro, M., Munoz, M. A. & Weninger, W. Targeting Rho-GTPases in immune cell migration and inflammation. British journal of pharmacology 171, 5491–5506, 10.1111/bph.12658 (2014).
Jin, Z. et al. Regulation of nuclear-cytoplasmic shuttling and function of Family with sequence similarity 13, member A (Fam13a) by B56-containing PP2As and Akt. Molecular biology of the cell, 10.1091/mbc.E14-08-1276 (2015).
Duluc, L. & Wojciak-Stothard, B. Rho GTPases in the regulation of pulmonary vascular barrier function. Cell and tissue research 355, 675–685, 10.1007/s00441-014-1805-0 (2014).
Corvol, H., Hodges, C. A., Drumm, M. L. & Guillot, L. Moving beyond genetics: is FAM13A a major biological contributor in lung physiology and chronic lung diseases? Journal of medical genetics 51, 646–649, 10.1136/jmedgenet-2014-102525 (2014).
Young, R. P. et al. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. The European respiratory journal 34, 380–386, 10.1183/09031936.00144208 (2009).
Maffettone, C., Chen, G., Drozdov, I., Ouzounis, C. & Pantopoulos, K. Tumorigenic properties of iron regulatory protein 2 (IRP2) mediated by its specific 73-amino acids insert. PloS one 5, e10163, 10.1371/journal.pone.0010163 (2010).
Falvella, F. S. et al. Transcription deregulation at the 15q25 locus in association with lung adenocarcinoma risk. Clinical cancer research: an official journal of the American Association for Cancer Research 15, 1837–1842, 10.1158/1078-0432.CCR-08-2107 (2009).
Liu, Y. et al. Haplotype and cell proliferation analyses of candidate lung cancer susceptibility genes on chromosome 15q24-25.1. Cancer research 69, 7844–7850, 10.1158/0008-5472.CAN-09-1833 (2009).
Siedlinski, M. et al. Dissecting direct and indirect genetic effects on chronic obstructive pulmonary disease (COPD) susceptibility. Human genetics 132, 431–441, 10.1007/s00439-012-1262-3 (2013).
Chappell, S. L. et al. The role of IREB2 and transforming growth factor beta-1 genetic variants in COPD: a replication case-control study. BMC medical genetics 12, 24, 10.1186/1471-2350-12-24 (2011).
Hardin, M. et al. CHRNA3/5, IREB2 and ADCY2 are associated with severe chronic obstructive pulmonary disease in Poland. American journal of respiratory cell and molecular biology 47, 203–208, 10.1165/rcmb.2012-0011OC (2012).
Ghio, A. J. et al. Particulate matter in cigarette smoke alters iron homeostasis to produce a biological effect. American journal of respiratory and critical care medicine 178, 1130–1138, 10.1164/rccm.200802-334OC (2008).
Guo, Y. et al. Genetic analysis of IREB2, FAM13A and XRCC5 variants in Chinese Han patients with chronic obstructive pulmonary disease. Biochemical and biophysical research communications 415, 284–287, 10.1016/j.bbrc.2011.10.042 (2011).
Wang, B. et al. Association of FAM13A polymorphisms with COPD and COPD-related phenotypes in Han Chinese. Clinical biochemistry 46, 1683–1688, 10.1016/j.clinbiochem.2013.07.013 (2013).
Chi, J. T. et al. Gene expression programs in response to hypoxia: cell type specificity and prognostic significance in human cancers. PLoS medicine 3, e47, 10.1371/journal.pmed.0030047 (2006).
Wright, J. M. et al. Respiratory epithelial gene expression in patients with mild and severe cystic fibrosis lung disease. American journal of respiratory cell and molecular biology 35, 327–336, 10.1165/rcmb.2005-0359OC (2006).
Hui, L., DelMonte, T. & Ranade, K. Genotyping using the TaqMan assay. Vol. Chapter 2 (2008).
Hochberg, Y. & Benjamini, Y. More powerful procedures for multiple significance testing. Statistics in medicine 9, 811–818 (1990).
Liyanarachchi, S. et al. Cumulative risk impact of five genetic variants associated with papillary thyroid carcinoma. Thyroid: official journal of the American Thyroid Association 23, 1532–1540, 10.1089/thy.2013.0102 (2013).
Acknowledgements
This study was supported by the National Science Centre, Poland, grant no. 2011/01/D/NZ5/02841. I. Ziółkowska-Suchanek is supported by the stipendium from the Foundation for Polish Science (START Program).
Author information
Authors and Affiliations
Contributions
I.Z.S. and M.M. designed methods and experiments, carried out the laboratory experiments, analyzed the data and interpreted the results. I.Z.S. drafted the manuscript. P.G., M.G., M.Ż., M.F. and E.S. participated in patient acquisition, collection of samples and statistical analyses. H.B.G., W.D. and J.N. made important intellectual contribution to the draft and revised the manuscript. All authors have read and approved the final version of the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Ziółkowska-Suchanek, I., Mosor, M., Gabryel, P. et al. Susceptibility loci in lung cancer and COPD: association of IREB2 and FAM13A with pulmonary diseases. Sci Rep 5, 13502 (2015). https://doi.org/10.1038/srep13502
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep13502
This article is cited by
-
FAM13A polymorphisms are associated with a specific susceptibility to clinical progression of oral cancer in alcohol drinkers
BMC Cancer (2023)
-
Family with sequence similarity 13 member A mediates TGF-β1-induced EMT in small airway epithelium of patients with chronic obstructive pulmonary disease
Respiratory Research (2021)
-
Toll-like receptor 4 (TLR4) expression is correlated with T2* iron deposition in response to doxorubicin treatment: cardiotoxicity risk assessment
Scientific Reports (2020)
-
Two-hybrid screening of FAM13A protein partners in lung epithelial cells
BMC Research Notes (2019)
-
What lies beneath? Molecular evolution during the radiation of caecilian amphibians
BMC Genomics (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.