Abstract
Accumulating evidence suggests a pivotal role of PDGFRß positive cells, a specific marker for central nervous system (CNS) pericytes, in tissue scarring. Identification of cells that contribute to tissue reorganization in the CNS upon injury is a crucial step to develop novel treatment strategies in regenerative medicine. It has been shown that pericytes contribute to scar formation in the spinal cord. It is further known that ischemia initially triggers pericyte loss in vivo, whilst brain trauma is capable of inducing pericyte detachment from cerebral vessels. These data point towards a significant role of pericytes in CNS injury. The temporal and spatial dynamics of PDGFRß cells and their responses in traumatic brain injury are poorly understood. Here we show that PDGFRß positive cells initially decline in the acute phase following experimental traumatic brain injury. However, PDGFRß positive cells increase significantly in the trauma zone days after brain injury. Using various pericyte markers we identify these cells to be pericytes that are demarcated by reactive gliosis. Our data indicate that brain trauma causes a biphasic response of pericytes in the early phase of brain trauma that may be of relevance for the understanding of pathological cellular responses in traumatic brain injury.
Similar content being viewed by others
Introduction
Pericytes play a pivotal role in a number of physiological and pathophysiological processes. They are of tremendous importance for vessel stability in various organ systems, e.g. the blood brain barrier (BBB) and in coronary vessels1,2, but also in tumor growth and vascularization3. Importantly, pericytes contribute to the formation of the neurovascular unit, which is comprised of brain endothelial cells, pericytes, astrocytes, neurons and glial cells4. Here, they are important in the regulation and development of the BBB5 and contribute to its proper function6. A loss of CNS pericytes induces BBB breakdown, neuroinflammation6 and CNS aneurysm formation7. In addition, pericytes are capable of regulating capillary diameter and microvascular perfusion within the CNS6,8,9.
In disease states such as cerebral ischemia, it has been shown that hypoxia causes pericyte death and persistent CNS microvascular obstruction8,10. We have previously shown a loss of pericytes in an in vitro preparation of the developing cerebral cortex upon ischemic and inflammatory stimuli11.
On the other hand, abnormal pericyte proliferation has been reported upon pathophysiological stimuli such as spine trauma and it is known that pericytes contribute to organ scar formation and tissue fibrosis12,13,14. Pericytes are further a promising cellular source for novel cellular therapy concepts. For example human pericytes have been reprogrammed recently into induced neuronal cells15,16. More than a decade ago, Dore-Duffy et al. have demonstrated that pericytes are capable of migrating away from the vessel wall after traumatic brain injury (TBI)32. Experimental data from a murine cerebral ischemia model17,18 have shown that platelet-derived growth factor receptor beta (PDGFRß), a highly specific marker for pericytes within the CNS19, has an important role in ischemic tissue responses upon injury. Fernández-Klett et al. have observed a significant increase in PDGFRß cellular mass formation within the ischemic core in a murine middle cerebral artery occlusion (MCAO) starting at days 3–5 after ischemic insult17. A better characterization of PDGFRß positive cell responses in TBI may therefore be of clinical importance in order to better understand and modulate TBI pathophysiology.
We were therefore interested in the temporal and spatial dynamics of PDGFRß positive cell formation following cortical controlled impact (CCI).
Material and Methods
Induction of CCI and ethical statement
C57/Bl6 mice were subjected towards CCI as described in detail elsewhere20. In order to detect CCI-induced cellular alterations, animals were compared with naïve mice in which the physiological arrangement of the neurovascular unit is preserved. In brief mice were anesthetized with isoflurane. Body temperature was monitored and maintained at 37 °C with the help of a heating pad throughout the experiment. For CCI a small skin incision above the skull was made and a burr hole was drilled. Then a precisely controlled pneumatic insult was applied above the right cortex with a custom made impactor (Supplementary Figure S1, diameter 3 mm, penetration depth 1.5 mm, velocity 8 m/s). After this procedure animals were allowed to recover and were clinically monitored regularly. Mice were sacrificed at several varying time points following injury and brains were collected for immunohistochemical processing or mRNA analyses (Fig. 1A). All experimental procedures were approved by the local ethical committee of the University Medical Center of the Johannes Gutenberg-University Mainz. The study protocol was approved by the Landesuntersuchungsamt RLP (protocol number: G12-1-010). All efforts were made to minimize the number of animals used and their suffering. European guidelines on the handling of animals were followed.
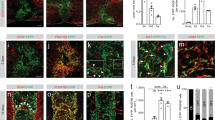
CCI results in pericontusional pericyte proliferation.
Tissue was collected for immunohistochemical and mRNA analyses at varying time points after induction of CCI (A). Under physiological conditions pericytes were located in a typical perivascular manner within the cerebral cortex (Claudin 5: green; PDGFRß: red). Only few cells were positive for Ki67, a marker for cell proliferation (B). Three days after CCI PDGFRß positive cells at the CCI border were associated with a strong increase of Ki67 immunoreactivity (C). Five days after CCI a prominent increase of PDGFRß expressing cells was detected (D). Panel E shows an orthogonal section view of a pericontusional pericyte positive for Ki67 that is not in contact with a microvessel. Quantification of PDGFRß staining demonstrates an initial loss of PDGFRß that is followed by a significant increase of PDGFRß 5 days after CCI (F). N = 5–6 animals per group; *P < 0.05; **P < 0.01.
Immunohistochemistry
Immunoreactive stainings were performed as documented before11,20. In brief brains were snap frozen in dry ice and cryosliced at 20 μm thickness (at Bregma −1 mm according to mouse brain atlas, mbl.org) with a cryostat (CryoStar™ NX70 Kryostat, Fischer Scientific). Care was taken to acquire sections from the same coordinates. Following cryoslicing probes were fixed in ice cold acetone for 5 minutes. Then tissue was further processed for immunostaining.Brain slices were blocked and permeabilized in 0.01 mol/l PBS containing 7% normal goat serum (Jackson Immuno Research Dianova, Hamburg, Germany) and 0.3% triton (Sigma-Aldrich Steinheim, Germany) for 2 hours. Then probes were incubated with primary antibodies in 2% bovine serum albumin (Dianova) with 0.05% azide and 0.1% Triton in 0.01 mol/l PBS overnight at room temperature. Subsequently probes were carefully washed with 0.01 mol/l PBS and then incubated with secondary antibodies in 2% bovine serum albumin for 2 hours at room temperature. After washing in 0.01 mol/l PBS sections were embedded in Fluoromount (Southern Biotech, Birmingham, AL, USA).
Table 1 and Table 2 give an overview on primary and secondary antibodies and dilutions used in this study.
Confocal imaging
Imaging was performed with a Leica SP5 confocal microscope using Leica Application Suite Advanced Fluorescence (Leica Microsystems). Excitations wavelengths were 405, 488, 561 and 633 nm. Care was taken to perform imaging with same parameters regarding exposure time and laser intensity for all samples.
Image analysis
Quantitative analyses were performed with NIH “Fiji is just Image J” (http://fiji.sc/Fiji). In order to quantify PDGFRß positive stained cells, Z-stacks were collected from each animal in the pericontusional zone (yellow region in Supplementary Figure S1). Z-stacks were Z projected at maximum intensity and binary images were created. Finally average gray scale intensity was acquired by executing the Fiji command “measure”. Details on the calculation of gray scale values by Fiji are documented at the NIH webpage: http://rsb.info.nih.gov/ij/docs/menus/analyze.html. Animated 3 dimensional reconstructions from acquired z-stacks were created using IMARIS (Bitplane, Switzerland).
Gene expression (mRNA) analysis
For time series analysis tissue preparation was performed as follows: Brains were quickly removed and placed in a cooled brain matrix (Zivic Instruments, Pittsburgh, USA). Perilesional injured brain tissue was immediately dissected, rapidly frozen in liquid nitrogen and stored at −80 °C until processing. Quantification of mRNA was performed by real-time polymerase chain reaction as described previously21. Absolute copy numbers of target genes were normalized against cyclophilin A (PPIA) as housekeeping gene22. Applied primers and probes are shown in Table 3. Same amounts of cDNA were amplified in duplicates using ABsolute™ Fast qPCR SYBR Green Mix (Thermo Fisher Scientific, Dreieich, Germany) for PPIA, PDGFRß, NG2 and Desmin according to the manufacturer’s instructions.
Statistics
For statistical analyses GraphPad Prism version 6.0 for Windows was used. All datasets were checked for normalization using the D’Agostino and Pearson method23. All datasets following a Gaussian distribution were analyzed using ONE WAY ANOVA followed by Dunnett’s test for multiple comparisons. Non-parametric data were analyzed with a Kruskal Wallis Test followed by a Dunns multiple comparison test. Differences were considered to be significant at alpha <0.05. Data are displayed as mean ± standard error of the mean.
Results
Impact of CCI on PDGFRß staining in the pericontusional zone
In the cerebral cortex of naïve control animals we found a physiological perivascular arrangement of PDGFRß positive cells. Under physiological conditions, only few cells were positive for the proliferation marker Ki67 (Fig. 1B). Three days after CCI the expression of the proliferation marker Ki67 increased within the pericontusional zone (Fig. 1C). Five days after CCI a significant cellular mass formation positive for the pericyte marker PDGFRß and Ki67 became apparent at the site of the lesion (Fig. 1D, inset E). High power imaging revealed PDGFRß positive cells that were immunoreactive for Ki67 (Fig. 1D). Many of these PDGFRß positive cells were not in contact with microvessels as documented in co-stainings with the microvascular marker Cl-5, which has been shown to be a specific marker for brain endothelial cells in brain trauma (Fig. 1E, Supplementary Movie 1)20,24.
Quantitative analyses revealed a significant decrease in PDGFRß staining 6–12 hours following CCI in the pericontusional zone (Fig. 1F). At 24 hours and 3 days post CCI no significant difference in PDGFRß positive cells could be detected when compared with naïve controls (Fig. 1F). However, on day 5 there was a significant increase of PDGFRß positive cells compared with controls (Fig. 1F).
Since PDGFRß is a specific marker for pericytes within the CNS, we further analyzed mRNA levels of pericyte markers on day 1, 3, 5 and 7 after CCI induction (Fig. 2A). Here we found that mRNA levels of the pericyte markers PDGFRß and NG2 were significantly upregulated after CCI with a peak on day 5 (Fig. 2B,C). The pericyte marker Desmin was already significantly upregulated on day 1 following CCI reaching its maximum mRNA levels on day 3 (Fig. 2D).
Pericyte differentiation markers are upregulated in a time dependent manner following CCI.
The pericyte expression markers PDGFRß, NG2 and Desmin were significantly upregulated following CCI. mRNA analyses were carried out on days 1, 3, 5 and 7 following TBI (A). PDGFRß mRNA levels were found to increase significantly, starting on day 3 after CCI with a peak at day 5 (B). The pericyte marker NG2 was also upregulated upon CCI from days 3–7 (C). Here, maximum NG2 mRNA levels were detected 5 days after brain trauma induction. The pericyte marker Desmin was already significantly upregulated on day 1, reaching its maximum expression levels on day 3 after CCI (D). N = 8–10 animals per group; *P < 0.05; **P < 0.01; ***P < 0.001.
PDGFRß positive cells in CCI are surrounded by a basement membrane
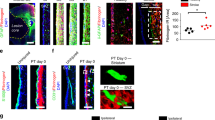
We and others have previously documented that PDGFRß is a specific marker for pericytes in the CNS11,19,25. Another property of pericytes is the presence of a basement membrane (BM) that maintains several laminin isoforms11,26. Association with a BM is an important criterion to identify pericytes27. By using a pan-laminin antibody, that recognizes several laminin isoforms, we observed that pericytes in intact cortices (Fig. 3A, Supplementary movie 2), as well as PDGFR beta positive cells in the pericontusional tissue after CCI are surrounded by a BM (Fig. 3B, Supplementary movie 3). Interestingly, pericytes in the pericontusional zone display an amoeboid like morphology that is significantly different from the perivascular morphology of pericytes in physiological conditions (Fig. 3A,B, Supplementary movies 2–5).
Pericytes in control and CCI conditions are sheathed by a basement membrane.
In order to verify the pericyte identity of PDGFRß positive cells, we performed pan-laminin co-stainings. In both, control (A) and CCI animals (B), PDGFRß positive cells were found to be sheathed by a basement membrane, a hallmark to identify pericytes.
PDGFRß positive cells express various pericyte differentiation markers
In order to further characterize PDGFRß positive cells in the pericontusional CCI zone, we performed co-stainings with a set of established pericyte differentiation markers such as NG2, Desmin and PDGFRß (Fig. 4). Here we found that PDGFRß co-localized with NG2 and partly with Desmin, indicating that PDGFRß cells were indeed pericytes (Fig. 4A,B). However there is not a 100 percent overlap between all markers. This is due to the different cellular localization of the respective proteins. PDGFRß is a transmembraneous tyrosine kinase, whilst Desmin is an intracellular intermediate filament protein28. NG2 is mostly expressed in a perinuclear manner in mature CNS pericytes that is also reflected by the immunofluorecent image in Fig. 4. However, we would like to point out that this discrepancy of marker overlap might also be partly due to a lack of specificity of these cellular markers.
PGDFRß cells in the pericontusional zone express the pericyte markers NG2 and Desmin.
Co-staining with the pericyte marker NG2 demonstrates that PDGFRß positive cells are immunoreactive for NG2 at day 5 after brain trauma (A). Note the typical perinuclear expression of NG2. In addition we found that some PDGFRß positive cells expressed the pericyte marker Desmin (B). Note that NG2 is a perinuclear protein whilst Desmin is an intracellular filament protein. Therefore both markers do not totally overlap with PDGFRß due to their different subcellular localization.
Reactive glia demarcates PDGFRß cell formation
It is well established that GFAP expressing reactive astrocytes are a major contributor to scar formation13,29 in the CNS. It has been recently documented in a model of murine ischemic stroke that proliferation of PDGFRß cells occurs in the ischemic core, which is demarcated by reactive astrogliosis25. In order to evaluate whether the observed PDGFRß cell proliferation is demarcated in a similar manner by reactive astrogliosis in CCI, we performed co-stainings with PDGFRß and the reactive astrocyte marker GFAP following CCI. In line with the literature we found that a high number of reactive astrocytes were present in the pericontusional zone. Similar to the results of Fernandez-Klett et al. in cerebral ischemia25, we found that Ki67 positive pericytes were demarcated by reactive astroglia 5 days after CCI (Fig. 5). In addition, PDGFRß and GFAP co-staining gave no evidence for an overlap of both immunohistochemical markers, indicating that PDGFRß and GFAP positive cells are distinct cell types.
Reactive astrocytosis demarcates proliferative PDGFRß cellular mass formation.
Immunofluorescent confocal analyses revealed that PDGFRß cells 5 days after CCI were a distinct cell population and different from reactive astrocytes. Co-stains for GFAP, a marker for reactive astrogliosis and PDGFRß demonstrate that reactive astrocytes demarcate a large number of proliferating PDGFRß positive cells. Note the high expression level of Ki67 in PDGFRß positive cells.
Discussion
Here we show in a murine model of CCI that pericytes undergo a biphasic cell response within the first 5 days after TBI.
A rapid loss of PDGFRß cells within the first 12 hours was followed by a significant increase of PDGFRß positive cells on day 5 after TBI. In our study PDGFRß immunoreactive cells expressed several pericyte markers such as Desmin and NG2 on protein level, pointing towards a pericyte identity. In addition, our qRT-PCR results on the increase of the pericyte markers NG2, Desmin and PDGFRß following TBI further support the immunofluorescence data.
However, the morphology of PDGFRß cells in CCI was different compared with physiological conditions. PDGFRß cells in the pericontusional TBI zone were amoeboid shaped and not necessarily in vasular contact. Co-stainings with GFAP revealed that PDGFRß cell mass formation was demarcated by reactive glia. Further these immunohistochemical results indicate that PDGFRß cells do not express GFAP, indicating they are not a subpopulation of reactive astrocytes.
Three to five days following brain trauma, Ki67 was found to be expressed by PDGFRß positive cells, which was not the case in physiological conditions. In line with our data on PDGFRß staining on day 5 after TBI, Fernández-Klett et al. have shown that PDGFRß positive cells significantly increase in a model of middle cerebral artery occlusion followed by reperfusion 5 days after stroke in mice25. In addition, the authors from that study also observed a demarcation of PDGFRß cell mass formation by reactive glia, which was also the case in our experimental study.
Interestingly, an enhanced cell proliferation was absent 2 weeks after brain trauma insult in a report by Chen et al.30. In our study we investigated proliferation for up to 5 days following trauma. Therefore our results may implicate that Ki67 is only transiently increased. However the trauma model used by Chen et al.30 is different to that used in our present study and the investigated species was rat whilst we examined C57/Bl6 mice. Our finding that pericytes play an important role in cellular brain injury responses is in line with data from Birbair et al. who have recently shown that 14 days after brain trauma, pericytes contribute to scar formation in the cortex14. However, in the study by Birbair et al. there was a mismatch in NG2 and PDGFRß expression 14 days after injury. Since we observed a colocalization of NG2 and PDGFRß immunoreactivity up to 5 days after injury, we speculate that the expression of both pericyte markers may undergo regulatory changes over longer time periods following TBI.
PDGFRß ablation experiments have shown that loss of PDGFRß induces pericyte loss, leading to an increase of BBB permeability, which results in extravasation of acute phase proteins into the CNS parenchyma that enhance secondary brain injury due to inflammation6,31. Therefore, therapeutic strategies to modulate PDGFRß related cell responses upon CNS injury may help to improve clinical outcome. We speculate that the observed biphasic response of PDGFRß positive cells within the first 5 days of TBI may present a promising target to modulate clinical complications such as evolution of brain edema or improve nerve fibre regeneration13,31.
In summary, our in vivo study identifies CNS pericytes as a cell population that declines in the acute phase of TBI, which is followed by reactive pericytosis on day 5 following cortical brain trauma. More research needs to be done in order to unravel the pathophysiological relevance of the observed biphasic pattern of PDGFRß cell dynamics.
Additional Information
How to cite this article: Zehendner, C. M. et al. Traumatic brain injury results in rapid pericyte loss followed by reactive pericytosis in the cerebral cortex. Sci. Rep. 5, 13497; doi: 10.1038/srep13497 (2015).
References
Chintalgattu, V. et al. Coronary Microvascular Pericytes Are the Cellular Target of Sunitinib Malate-Induced Cardiotoxicity. Sci. Transl. Med. 5, 187ra69–187ra69 (2013).
Winkler, E. A., Bell, R. D. & Zlokovic, B. V. Central nervous system pericytes in health and disease. Nat. Neurosci. 14, 1398–1405 (2011).
Cheng, L. et al. Glioblastoma Stem Cells Generate Vascular Pericytes to Support Vessel Function and Tumor Growth. Cell 153, 139–152 (2013).
Zlokovic, B. V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57, 178–201 (2008).
Armulik, A. et al. Pericytes regulate the blood–brain barrier. Nature 468, 557–561 (2010).
Bell, R. D. et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68, 409–427 (2010).
Lindahl, P. Pericyte Loss and Microaneurysm Formation in PDGF-B-Deficient Mice. Science 277, 242–245 (1997).
Hall, C. N. et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 508, 55–60 (2014).
Peppiatt, C. M., Howarth, C., Mobbs, P. & Attwell, D. Bidirectional control of CNS capillary diameter by pericytes. Nature 443, 700–704 (2006).
Yemisci, M. et al. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat. Med. 15, 1031–1037 (2009).
Zehendner, C. M., Wedler, H. E. & Luhmann, H. J. A novel in vitro model to study pericytes in the neurovascular unit of the developing cortex. PloS One 8, e81637 (2013).
Chen, Y.-T. et al. Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int. 80, 1170–1181 (2011).
Goritz, C. et al. A Pericyte Origin of Spinal Cord Scar Tissue. Science 333, 238–242 (2011).
Birbrair, A. et al. Type-1 pericytes participate in fibrous tissue deposition in aged skeletal muscle. AJP Cell Physiol. 305, C1098–C1113 (2013).
Karow, M. Mountaineering pericytes - A universal key to tissue repair? BioEssays News Rev. Mol. Cell. Dev. Biol. (2013) 10.1002/bies.201300055.
Karow, M. et al. Reprogramming of Pericyte-Derived Cells of the Adult Human Brain into Induced Neuronal Cells. Cell Stem Cell 11, 471–476 (2012).
Fernández-Klett, F. et al. Early loss of pericytes and perivascular stromal cell-induced scar formation after stroke. J. Cereb. Blood Flow Metab. 33, 428–439 (2012).
Shen, J. et al. PDGFR-β as a positive regulator of tissue repair in a mouse model of focal cerebral ischemia. J. Cereb. Blood Flow Metab. 32, 353–367 (2011).
Winkler, E. A., Bell, R. D. & Zlokovic, B. V. Pericyte-specific expression of PDGF beta receptor in mouse models with normal and deficient PDGF beta receptor signaling. Mol. Neurodegener 5, 32 (2010).
Thal, S. C. et al. Volatile Anesthetics Influence Blood-Brain Barrier Integrity by Modulation of Tight Junction Protein Expression in Traumatic Brain Injury. PLoS ONE 7, e50752 (2012).
Thal, S. C. et al. Inhibition of proteasomal glucocorticoid receptor degradation restores dexamethasone-mediated stabilization of the blood-brain barrier after traumatic brain injury. Crit. Care Med. 41, 1305–1315 (2013).
Timaru-Kast, R., Herbig, E. L., Luh, C., Engelhard, K. & Thal, S. C. Influence of age on cerebral housekeeping gene expression for normalization of quantitative PCR after acute brain injury in mice. J. Neurotrauma (2015). 10.1089/neu.2014.3784.
D’Agostino, R. B. & Pearson, E. S. Testing for Departures From Normality. 44, 316–321 (1990).
Campbell, M. et al. Targeted suppression of claudin-5 decreases cerebral oedema and improves cognitive outcome following traumatic brain injury. Nat. Commun. 3, 849 (2012).
Fernández-Klett, F. et al. Early loss of pericytes and perivascular stromal cell-induced scar formation after stroke. J. Cereb. Blood Flow Metab. 33, 428–439 (2012).
Sixt, M. et al. Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood-brain barrier in experimental autoimmune encephalomyelitis. J. Cell Biol. 153, 933–946 (2001).
Krueger, M. & Bechmann, I. CNS pericytes: concepts, misconceptions and a way out. Glia 58, 1–10 (2010).
Armulik, A., Genové, G. & Betsholtz, C. Pericytes: Developmental, Physiological and Pathological Perspectives, Problems and Promises. Dev. Cell 21, 193–215 (2011).
Sofroniew, M. V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 32, 638–647 (2009).
Chen, X.-H., Iwata, A., Nonaka, M., Browne, K. D. & Smith, D. H. Neurogenesis and glial proliferation persist for at least one year in the subventricular zone following brain trauma in rats. J. Neurotrauma 20, 623–631 (2003).
Winkler, E. A., Sengillo, J. D., Bell, R. D., Wang, J. & Zlokovic, B. V. Blood–spinal cord barrier pericyte reductions contribute to increased capillary permeability. J. Cereb. Blood Flow Metab. 32, 1841–1852 (2012).
Dore-Duffy, P. et al. Pericyte migration from the vascular wall in response to traumatic brain injury. Microvasc. Res. 60, 55–69 (2000).
Acknowledgements
We thank Beate Krumm for excellent technical assistance. This study was supported by DFG grants to HJL and SCT. CMZ received support from: the Johannes Gutenberg-University of Mainz (Start up grant), ECCPS clinical career grant (DFG), LOEWE Cell and Gene Therapy grant (State of Hesse) and a start up grant from the Goethe University Frankfurt. FB is supported by a scholarship of the DFG (SFB 834). We thank the IMB Core Facility Microscopy of the Institute of Molecular Biology (IMB) in Mainz for confocal image acquisition.
Author information
Authors and Affiliations
Contributions
C.M.Z. and S.C.T. designed the study, performed experiments and analyzed the data, C.M.Z. and H.L. wrote the main manuscript, A.S., F.B. and A.H. performed experiments, C.M.Z. designed all figures, C.M.Z. designed 3 dimensional reconstructions, S.C.T. designed supplementary figure 1.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zehendner, C., Sebastiani, A., Hugonnet, A. et al. Traumatic brain injury results in rapid pericyte loss followed by reactive pericytosis in the cerebral cortex. Sci Rep 5, 13497 (2015). https://doi.org/10.1038/srep13497
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep13497
This article is cited by
-
Mechanism of COVID-19-Induced Cardiac Damage from Patient, In Vitro and Animal Studies
Current Heart Failure Reports (2023)
-
Chronic effects of blast injury on the microvasculature in a transgenic mouse model of Alzheimer’s disease related Aβ amyloidosis
Fluids and Barriers of the CNS (2022)
-
Emerging roles for CNS fibroblasts in health, injury and disease
Nature Reviews Neuroscience (2022)
-
Structural disruption of the blood–brain barrier in repetitive primary blast injury
Fluids and Barriers of the CNS (2021)
-
Analyses of the pericyte transcriptome in ischemic skeletal muscles
Stem Cell Research & Therapy (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.