Abstract
The Nymphoides peltata (N. peltata) population has shown rapid expansion in Lake Taihu, China, in recent years. The core question is whether N. peltata seeds have contributed to the expansion. To address this, we randomly selected three N. peltata stands to investigate the seed bank characteristics of N. peltata in Lake Taihu. Results showed that N. peltata had high seed production, with a maximum seed yield of 1763 seeds per m2. Density of intact and fragmented seeds decreased rapidly with sediment depth. Few intact or fragmented seeds were distributed at depths greater than 4 cm in the sediment. Spatial distribution of the seed bank indicated that most seeds sank to the sediment within the N. peltata stands and few seeds took advantage of their floating ability. Seeds recovered from the sediment during April to June had a low germination rate and no seeds germinated during October to April. Cold exposure treatment increased the germination rate remarkably. No seedlings were found in the field from January 2012 to December 2012, indicating that few seeds were successfully established in the surveyed area. The results suggested that sexual reproduction had little direct contribution to the N. peltata expansion in this large shallow lake.
Similar content being viewed by others
Introduction
Nymphoides peltata is a common and relatively widespread aquatic plant1. In the past few decades, N. peltata has become an invasive pest species in North America and New Zealand2,3. In China, this native species has rapidly expanded in waterways and lakes, such as Lake Taihu in Jiangsu Province. This species tends to grow in dense patches4,5, which not only threatens recreational vessels, but also lead to stagnant areas with low oxygen levels6 and the competitive exclusion of other submerged macrophytes with overwhelming superiority7.
Nymphoides peltata propagates by vegetative and sexual means8. The small-sized seeds can float on the water surface, which should contribute to long distance dispersal. The ecology of N. peltata seeds has been studied due to the quick expansion of N. peltata populations in many lakes. Previous research has described development progress from the flower bud to fruit, with the mean number of developed seeds per m2 calculated to be 9434 in an experimental tank (S.D. = 5668, n = 4)9. However, studies on the production of fruits and seeds in the field remain limited10,11. When fruits are well developed, seeds are released and float on water by holding a hydrophobic surface8, which could be beneficial for efficient dispersal. Van der Velde and Van der Heijden9 reported that seeds remained floating in undisturbed Petri dishes after two months, though other studies have shown that most seeds were immediately submerged under the interference of simulated rain12. How far N. peltata seeds can float in the field remains unclear and knowledge on the spatial distribution of seed density in sediment inside and outside wild N. peltata population stands is also limited.
After being shed from parent plants, mature seeds eventually settle to the soil surface and form a seed bank13. Viable seeds present by the end of the germination period and the period of seed release is considered a persistent seed bank12,14,15. In an unpredictable environment, a persistent seed bank is a mechanism to buffer the effect of environmental variability13 and thus the existence of a persistent seed bank prevents the risk of extinction through random fluctuations and plays an important role in population recruitment16. Although N. peltata has expanded quickly and has many negative effects on the native ecosystem, little work had been undertaken on the seed bank characteristics of N. peltata, which is essential for better understanding the contribution of sexual recruitment to the expansion of N. peltata populations.
Seed germination is a vital step in the process of population establishment. Takagawa et al.17 reported that safe-sites for N. peltata seed germination were less prone to inundation and were exposed to sufficient light during the spring water-level drawdown. However, seeds located far from the shoreline might be faced with different conditions, such as low oxygen concentration and high sediment deposition rate. After seed germination, successful seedling establishment is also important for population recruitment.
In this paper, we hypothesized that sexual reproduction may play an important role in the expansion of the N. peltata population in the open waters of Lake Taihu. Thus, several aspects were investigated in Lake Taihu, including (1) seed yields; (2) spatial distribution of the seed bank inside and outside N. peltata population stands; (3) annual dynamics of the seed bank; and (4) seed germination and seedling density in N. peltata stands. These experiments were expected to provide a detailed understanding on the function of sexual recruitment in N. peltata population expansion in Lake Taihu.
Results
Seed yield
The reproductive organ development patterns differed between the A, B and C communities (Table 1). Flower density and bud density per inflorescence were larger in B and C than in A, while the fruit density per inflorescence was largest in A. This indicated that stand A flowered earlier than the other two stands. There was also great variation in the maximum seed yield per m2 among the three stands, with a range of 428 to 1763 seeds per m2.
Spatial and temporal distribution of the seed bank
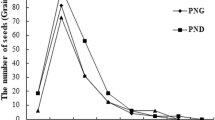
Vertical distribution of seeds at the three sites showed a similar pattern (Fig. 1). The N. peltata seeds were mainly distributed on the sediment surface in September 2011. The number of intact and fragmented seeds decreased rapidly with sediment depth. Few intact seeds or seed fragments were distributed at depths greater than 4 cm in all three N. peltata stands. These results suggested that a sediment depth up to 10 cm would accurately determine N. peltata seed density in this study area.
As seen from Figs 2 and 3, considerable variation existed in seed banks between the three stands. However, the seasonal dynamics of seed banks had a similar feature. The N. peltata seed bank increased from October 2011 to November 2011, then decreased gradually from December 2011 to March 2012 and reached to a relatively steady level of 123 ± 101 (mean ± S.D., n = 27) seeds per m2 in May 2012. After May 2012, however, the seed bank increased rapidly, with a maximum of 908 ± 490 (mean ± S.D., n = 10) seeds per m2. The temporal variation in seed fragment density in the present study was similar to that of intact seeds.
Seed density had a maximum value of 263 ± 145 seeds per m2 in the center of the community of stand B and decreased sharply along the transects (Fig. 4). The seed density in the center was six times that of the density at the edge of the community. Seed density was low in sites 10 m from the community edge. No seed was found in sediment cores in sites 20 m from the edge of the N. peltata community.
Seedling dynamics and germination
From January 2012 to December 2012, seedling densities were estimated monthly by counting seedlings in the field. However, no N. peltata seedling was found when investigating the seed bank in the field.
The germination rate of seeds recovered from the sediment was low and decreased with time. Seed germination rates were 21.1%, 18.9% and 11.1% in April, May and June, respectively. Cold exposure treatment increased the germination rate remarkably. Cumulative germination rates were 73.3%, 74.4% and 76.7% in April, May and June, respectively. Seed bag experiments showed that no seed germinated below or above the sediment in Lake Taihu. Non-germinated seeds were stained with tetrazolium, which showed they were viable.
Discussion
The sizes of the three investigated stands were similar with a radius of around 60 m. The coverage of stands A, B and C were 100%, 100% and 80%, respectively. Accompanying species were Vallisneria natans and Hydrilla verticillata in site A and B, Potamogeton malaianus in site C. Great differences at reproductive stages were observed between the three N. peltata stands in Lake Taihu. N. peltata flowered earlier in site A than in sites B and C. One possible reason was the difference in sediment type: very dark silt in site A compared with shallow silt-loam in sites B and C. Sediment is considered as the main nutrient source for rooted macrophytes18,19 and can affect macrophyte development. In Nijmegen (Netherlands), Van der Velde and Van der Heijden9 reported that the mean number of fruits per m2 was 180 (S.D. = 76.3, n = 25), with an average of 26.5 seeds per fruit. They also calculated the average number of developed seeds per m2 to be 3117 in a N. peltata stand. The results of the present study revealed that N. peltata had a smaller seed number per m2 in Lake Taihu, probably due to the different climate and eutrophic level of water.
Seeds were found mostly concentrated in the top sediment layer, in agreement with previous studies20,21. This is possibly due to seed longevity. Thompson22 suggested that small, round compact seeds are persistent in soil, while large, flat seeds are often short lived. Since the seeds of N. peltata are flat, broad-elliptical and thick4,9, they may be transient in sediment. Therefore, seed density decreased sharply with sediment depth.
The temporal pattern of intact seed density in sediment indicated that many fresh seeds fell to the sediment and become part of the seed bank from October 2011 to November 2011. After that, the seed number experienced a net reduction from December 2011 to May 2012, which was probably caused by seed emergence and feeding by animals8. After May 2012, reproductive organs began to develop, followed by many seeds being released from mature fruits and settling to the sediment surface. In addition, seeds would not germinate without exposure to cold temperature11 and seed consumption by animals may be limited8, which led to the rapid increase in seed density from May 2012. Temporal dynamics of seed fragment density was similar to that of intact seed density, which indicated that most seed fragments decomposed rapidly. As seen in Fig. 2, a persistent seed bank occurred in N. peltata stands, which is vital for population recruitment13. Existence of a persistent seed bank prevents the risk of extinction through unexpected disasters16. It is supposed that it takes several years to exhaust seed banks through seedling emergence23. If N. peltata cannot produce seeds under poor environmental conditions and few asexual propagates are conserved, sexual reproduction may recruit the population and improve the stability of the N. peltata community.
Dispersal ability plays an important role in population dynamics. Van der Velde and Van der Heijden9 evaluated the floating ability of N. peltata seeds and reported that N. peltata seeds remained floating in Petri dishes for two months without any disturbance. However, the results of spatial distribution in the present study indicated that most seeds sank quickly to the sediment within the N. peltata stands. One possible reason might be that N. peltata populations usually form dense patches with high leaf index area in lakes10. When ripe seeds are released from fruits, the floating seeds may encounter numerous leaves on the water surface, which may break the hydrophobic coating outside the seeds8. As a result, seeds would sink quickly within the stand. Seeds released at the edge of the stand are more likely to be the source of long-distance dispersal.
The results showed evidence that few N. peltata seedlings were established in the study area of Lake Taihu. Previous studies also suggest that only a small area of the lake has safe sites for N. peltata seedling establishment17,24. Even if some N. peltata seeds can germinate, most detach from the sediment and float to the water surface4,25.
Seed germination experiments revealed that only a small proportion of seeds germinated readily when collected from sediment during April to June. Seed dormancy can be divided into physical and physiological (innate) dormancy. The geminated seeds may be categorized as physically dormant26, since N. peltata seeds were unable to germinate when they were under hypoxic conditions in the sediment4,11. Most of the non-germinated seeds were innately dormant, with four weeks cold exposure overcoming their physiological dormancy, indicating that the natural cold exposure period was not long enough in winter and further cold treatment was needed to break seed dormancy in the following year in Lake Taihu. The non-germinated seeds were gradually covered by sediment until the next year and physiologically dormant seeds probably became physically dormant, which might inhibit successful germination.
Previous studies indicate that N. peltata expands locally with runners. If the runners have developed roots that are not yet attached to the sediment and the runners are broken by natural forces, vegetative fragments will be released and eventually dispersed to favourable areas where they can establish themselves3,9. Recently, Larson used molecular markers to investigate reproduction strategy of N. peltata in Sweden27. Lacking of genetic variation suggested that vegetative reproduction constituted an important part of the total reproduction27. These literatures indirectly confirm that sexual reproduction may play a minor role in population expansion.
Conclusion
This study suggested that N. peltata could produce a large number of seeds in Lake Taihu. Most seeds sank within the stand and a large proportion of the seeds were innately dormant, with no seedlings found in the study area. Sexual reproduction contributed little to the rapid expansion of N. peltata in the open waters of this large shallow lake. The expansion of N. peltata populations was probably maintained by the dispersal of vegetative fragments.
Methods
Study site
Lake Taihu is a shallow lake with a surface area of 2,338 km2 and a mean depth of 1.9 m. In the 1950s, the lake was oligotrophic. Since the 1980s, however, water quality has continuously deteriorated and the lake is now eutrophic. Blue-green algae dominate the western part of the lake, whilst the eastern part is mainly covered by vascular plants5,28. Satellite images of Lake Taihu at different periods revealed that the population of hydrophytes has been increasing rapidly since 2001 and floating-leaved macrophytes dominate the lake, covering a surface area of 89.1 km2 in 20045. According to field surveys conducted in the summers of 2004 and 2013, N. peltata is the dominant floating-leaved macrophyte in Lake Taihu4. To investigate the seed characteristics of N. peltata, three N. peltata stands were carefully selected for our study (Fig. 5), which were denoted as A (31.12070° N, 120.39241° E), B (31.12295° N, 120.38566° E) and C (31.12460° N, 120.39346° E). In each stand, N. peltata has been colonized for more than ten years. The three N. peltata stands we chose were isolated to each other and the shapes of the three stands were almost round; while most other stands were closed to each other, making it difficult to investigate the spatial distribution of seed and to assess the dispersal ability. In addition, according to our previous field survey conducted in the summer of 2004, 2008 and 2010, most sediment colonized by N. peltata was silt. The sediments of site A, B and C were all silt. Therefore, It was representative to choose the three sites as the study place.
Estimation of seed yield
The field investigation showed that the growing season of N. peltata ranged from April to late October. Blooming began in late May or early June. Peak blooming occurred from August to October and a few flowers continued emerging until November in Lake Taihu. This was similar to the flowering period reported in the Netherlands9. To estimate the seed yield of N. peltata, 15 quadrats (1 m2) were randomly established in the three stands on September 1, 2012. The inflorescence number in each quadrat as well as fruit, flower and flower bud numbers per inflorescence were recorded. The average seed number of 40 randomly selected fruits from each community was counted. Maximum seed yield per m2 was estimated by multiplying the sum of bud number, flower number, fruit number and inflorescence number and average seed number.
Characterizing spatial-temporal distribution of the seed bank
Seed separation was used to examine the N. peltata seed bank. Seed separation utilizes the differences in size or density to separate seeds from sediment29. Since the mean length and width of the N. peltata seeds are 3.8–5.1 mm and 2.7–3.0 mm, respectively9, it was possible to detect seeds efficiently from the sediment. In the study, the seeds were classified into two categories: intact seeds and seed fragments. Intact seeds were defined as seeds having intact and hard seed capsules with or without marginal bristles12; seed fragments included fragmented seed capsules with marginal bristles and decayed seeds.
The vertical distribution of the seeds was first investigated to estimate the N. peltata seed bank in Lake Taihu. Three replicate sediment cores (diameter 10 cm, length 25 cm) were taken randomly in the centers of the three N. peltata stands on September 1, 2011. Cores were immediately sliced into 2 cm sections from the surface to a 20 cm depth. Each section was put into a plastic bag and transported to the laboratory. The sediments were concentrated by washing with a gentle water flow through a fine sieve (0.425 mm mesh) and both the stone roots and vegetative parts were removed. The mesh was small enough to catch N. peltata seeds. The seeds were then selected using the seed separation method.
The spatial distribution of N. peltata seeds was investigated to reveal seed floating capacity. Stand B was chosen as the study site because the N. peltata community had a regular circle shape with a radius of 60 m and there were no other N. peltata stands within 200 m. Sampling transects were made in four directions (east, south, west and north) from the center of N. peltata community. Field investigation showed that there was no seed in sediment 30 m from the edge of the community. Therefore, the farthest sample point was 20 m from the community edge. In addition, there were two other sampling points in each transect located at the edge of the community and 10 m from the community edge. Sediment cores were collected along established transects from the center of the N. peltata stand B. Nine replicate sediment cores (diameter 10 cm, length 25 cm) were taken in October 2012. The seed selection procedure followed the method described above.
Estimation of seedling dynamic and germination
Seedling densities in the field were estimated by counting seedlings monthly from January to December 2012. An Ekman sediment sampler (15 cm × 15 cm × 20 cm) was employed to examine the density of the seedlings. Nine replicate samples were randomly taken in each of the three N. peltata stands. A water depth of approximately 10 cm was maintained over the sediment surface during the sampling process to avoid any disturbance of the sediment. The N. peltata seedlings were examined and counted.
The seeds picked from sediment cores in sites A, B and C from April to June in 2012 were used for the seed germination experiment. Seeds collected from the three communities were well mixed. Batches of 30 seeds were used and experiments were executed in triplicate. Seeds were incubated in a Petri dish filled with fresh tap water (0.3 mm depth) in which all seeds were submerged. Light was provided by a fluorescent white tube. Each treatment was tested at 20 μEinstein m−2 s−1 with a photoperiod of 14 h. All experiments were conducted at 25 °C in a room for a period of 4 weeks. Germinated seeds were counted and removed every other day and tap water was replenished weekly. Another experiment was conducted to evaluate the effect of cold stratification (here defined as storage in demineralized water at 4 °C) on the seed germination rate. After incubation at 25 °C for 4 weeks, the remaining non-germinated seeds were stored in a refrigerator for another 4 weeks at 4 °C. The seeds were then incubated in the same conditions as above. During the experiment, if the radicle protruded at least 1 mm from the seed, the seed was scored as germinated.
To study the seed germination process in the field, seed bag experiments were performed. Seeds were collected from mature fruits and placed in nylon mesh bags (0.5 mm mesh, 4.0 cm × 5.0 cm) on October 10, 2012. Each bag contained 50 seeds. Five bags were attached to an iron peg, which was pushed into the sediment to keep the seed bags buried at a depth of approximately 2 cm. To mimic the aboveground seed bank, the five bags were connected to the iron peg by a nylon line and each bag contained a float ball to avoid the bags being covered by sediment. In May 2013, these bags were harvested to evaluate germination. To examine the viability of the seeds that did not germinate, the seeds were cut and the embryos were treated with tetrazolium solution30.
Additional Information
How to cite this article: Huang, W. et al. Seed bank characteristics of the Nymphoides peltatapopulation in Lake Taihu. Sci. Rep. 5, 13261; doi: 10.1038/srep13261 (2015).
References
Li, Z. Q., Xu, J., Cao, T., Ni, L. Y. & Xie, P. Adaptive responses of a floating-leaved macrophyte, Nymphoides peltata, to a terrestrial habitat. J. Freshw. Ecol. 25, 481–486 (2010).
Champion, P. D. & Clayton, J. S. The evaluation and management of aquatic weeds in New Zealand in Plant Invasions: Ecological Threats and Management Solutions (eds. Child, L. et al. ) 429–434 (Backhuys, 2003).
Darbyshire, S. J. & Francis, A. The biology of invasive alien plants in Canada. 10. Nymphoides peltata (S. G. Gmel.) Kuntze. Can. J. Plant Sci. 88, 811–829 (2008).
Huang, W., Chen, K. N., Shi, X., Ren, K. X. & Li, W. C. The contribution of seeds to the recruitment of a Nymphoides peltata population. Limnologica—Ecology and Management of Inland Waters 44, 1–8 (2014).
Ma, R., Duan, H., Gu, X. & Zhang, S. Detecting aquatic vegetation changes in Taihu Lake, China using multi-temporal satellite imagery. Sensors 8, 3988–4005 (2008).
Caraco, N., Cole, J., Findlay, S. & Wigand, C. Vascular plants as engineers of oxygen in aquatic systems. Bioscience 56, 219–225 (2006).
Wu, Z. H. & Yu, D. The effects of competition on growth and biomass allocation in Nymphoides Peltata (Gmel.) O. Kuntze growing in microcosm. Hydrobiologia 527, 241–250 (2004).
Cook, C. D. K. Seed dispersal of Nymphoides peltata (S. G. Gmelin) O. Kuntze (Menyanthaceae). Aquat. Bot. 37, 325–340 (1990).
van der Velde, G. & van der Heijden, L. A. The floral biology and seed production of Nymphoides peltata (Gmel) O. Kuntze (Menyanthaceae). Aquat. Bot. 10, 261–293 (1981).
Brock, T. C. M., Arts, G. H. P., Goossen, I. L. M. & Rutenfrans, A. H. M. Structure and annual biomass production of Nymphoides peltata (Gmel) Kuntze,O. (Menyanthaceae). Aquat. Bot. 17, 167–188 (1983).
Smits, A. J. M., van Avesaath, P. H. & van der Velde, G. Germination requirements and seed banks of some nymphaeid macrophytes: Nymphaea alba L. Nuphar lutea (L.) Sm. and Nymphoides peltata (Gmel.) O. Kuntze. Freshw. Biol. 24, 315–326 (1990).
Smits, A. J. M., van Ruremonde, R. & van der velde, G. Seed dispersal of three nymphaeid macrophytes. Aquat. Bot. 35, 167–180 (1989).
Fenner, M. & Thompson, K. The Ecology of Seeds. (Cambridge University Press, 2005).
Baskin, C. C. & Baskin, J. M. Seeds: Ecology, Biogeography and Evolution of Dormancy and Germination. (Academic Press, 1998).
Grime, J. P. Plant strategies, vegetation processes and ecosystem properties. (John Wiley and Sons, 2001).
Warner, R. R. & Chesson, P. L. Coexistence mediated by recruitment fluctuations: a field guide to the storage effect. Am. Nat. 125, 769–787 (1985).
Takagawa, S., Nishihiro, J. & Washitani, I. Safe sites for establishment of Nymphoides peltata seedlings for recovering the population from the soil seed bank. Ecol. Res. 20, 661–667 (2005).
Barko, J. W. & Smart, R. M. Sediment-based nutrition of submersed macrophytes. Aquat. Bot. 10, 339–352 (1981).
Carignan, R. & Kalff, J. Phosphorus sources for aquatic weeds: Water or sediments? Science 207, 987–989 (1980).
Bekker, R. M. et al. Seed size, shape and vertical distribution in the soil: indicators of seed longevity. Funct. Ecol. 12, 834–842 (1998).
Bonis, A. & Lepart, J. Vertical structure of seed banks and the impact of depth of burial on recruitment in two temporary marshes. Vegetatio 112, 127–139 (1994).
Thompson, K., Band, S. R. & Hodgson, J. G. Seed size and shape predict persistence in soil. Funct. Ecol. 7, 236–241 (1993).
Welling, C. H. & Becker, R. L. Seed bank dynamics of Lythrum salicaria L.: implications for control of this species in North America. Aquat. Bot. 38, 303–309 (1990).
Nishihiro, J., Kawaguchi, H., Iijima, H., Fujiwara, N. & Washitani, I. Conservation ecological study of Nymphoides peltata in Lake Kasumigaura. Ecology and Civil Engineering 4, 39–48 (2001).
Hutchinson, G. E. A Treatise on Limnology: Volume III. Limnological botany. (John Wiley & Sons, 1975).
Baskin, J. M. & Baskin, C. C. A classification system for seed dormancy. Seed Sci. Res. 14, 1–16 (2004).
Larson, D. Reproduction strategies in introduced Nymphoides peltata populations revealed by genetic markers. Aquat. Bot. 86, 402–406 (2007).
Qin, B. Q., Xu, P. Z., Wu, Q. L., Luo, L. C. & Zhang, Y. L. Environmental issues of Lake Taihu, China. Hydrobiologia 581, 3–14 (2007).
Roberts, H. A. Seed banks in soils. Advances in applied biology 6, 1–55 (1981).
Moore, R. P. Tetrazolium staining for assessing seed quality in Seed Ecology: Proceedings of the Nineteenth Easter School in Agricultural Science (ed. Heydecker, W. ) 347–366 (University of Nottingham, 1973).
Acknowledgements
The authors greatly thank help from Dr. Xiang Bai, Ms. Kuixiao Ren and Xin Yang during our fieldwork in Lake Taihu. This research was supported by the National Natural Science Foundation of China (No. 41171413 and No. 51425902).
Author information
Authors and Affiliations
Contributions
W.H., Q.C. and K.C. devised the original concept, designed the experiment, discussed the interpretation of results and co-wrote the paper. W.H. and K.C. performed the experiments and analyzed the data. W.H. prepared the draft manuscript and Q.C. made the final version. All authors reviewed and proved the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Huang, W., Chen, Q. & Chen, K. Seed bank characteristics of the Nymphoides peltata population in Lake Taihu. Sci Rep 5, 13261 (2015). https://doi.org/10.1038/srep13261
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep13261
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.