Abstract
The formation of proper sensory afferent connections during development is essential for brain function. Activity-based competition is believed to drive ocular dominance columns (ODC) in mammals and in experimentally-generated three-eyed frogs. ODC formation is thus a compromise of activity differences between two eyes and similar molecular cues. To gauge the generality of graphical map formation in the brain, we investigated the inner ear projection, known for its well-defined and early segregation of afferents from vestibular and auditory endorgans. In analogy to three eyed-frogs, we generated three-eared frogs to assess to what extent vestibular afferents from two adjacent ears could segregate. Donor ears were transplanted either in the native orientation or rotated by 90 degrees. These manipulations should result in either similar or different induced activity between both ears, respectively. Three-eared frogs with normal orientation showed normal swimming whereas those with a rotated third ear showed aberrant behaviors. Projection studies revealed that only afferents from the rotated ears segregated from those from the native ear within the vestibular nucleus, resembling the ocular dominance columns formed in three-eyed frogs. Vestibular segregation suggests that mechanisms comparable to those operating in the ODC formation of the visual system may act on vestibular projection refinements.
Similar content being viewed by others
Introduction
The function of the central nervous system hinges on the specific connection of sensory afferents to provide the brain with information about the environment to react to sensory stimuli. The details of forming such connections are understood down to the molecular level in the visual system1 and the olfactory system2,3,4 but are less clear in the inner ear. This deficiency is partly owing to the ear's complexity, with three sensory systems housed in one organ5. While the vestibular sensory neurons project partially overlapping gravistatic and angular acceleration information into the vestibular nuclei complex6, the spiral ganglion project auditory information to cochlear nuclei. Projection to the cochlear nuclei is possibly specified by transcription factors such as Gata35 and Neurod17. It has been shown that activity fine tunes the temporal pattern of projections to the cochlear nuclei8, though the amount of segregation in the cochlear nuclei prior to activity in the cochlea has not been thoroughly investigated at early stages9. Based on stereotyped projections prior to hair cell development10, it seems likely that inner ear vestibular sensory projections are molecularly targeted to vestibular nuclei11 whereas the partial overlapping and partial segregated projection of different sensory organ afferents within the target area6,12,13 may be later driven by differential activity. Activity-mediated partial segregation of overlapping afferents is well known in the visual system as ocular dominance columns (ODCs). ODCs appear either naturally in animals with binocular vision14,15 or can be induced through grafting of a third eye16. It is thought that ODC formation is a consequence of common molecular targeting and differential spontaneous or induced activity17,18. Indeed, blocking electric activity or synaptic transmission disrupts formation of ODCs19, indicating that in the absence of activity the compromise between molecular guidance and activity is shifted toward the molecular cues.
In addition to providing a better understanding of activity-mediated segregation of afferents onto target neurons18, transplantation experiments have expanded our understanding of afferent target interaction and selection. In the olfactory system transplanted olfactory afferents transform any brain area they reach into olfactory glomeruli-like organizations20,21,22,23,24. Misrouted retinal ganglion cell axons of transplanted eyes show a surprising ability to form functional connections, even if they enter the spinal cord25. In contrast, inner ear projections into foreign parts of the brain show random distribution with no stereotyped pattern emerging26. Afferents of ears transplanted to the orbit grow into various brain areas along the oculomotor, optic, or trigeminal nerves26. Afferents entering the midbrain along the oculomotor or optic nerves never reach the vestibular nuclei, indicating absence of long attraction to lure fibers to the vestibular nuclei of the hindbrain. However, afferents reaching the hindbrain along the trigeminal nerve tend to aggregate in the vestibular nuclei through unknown, possibly short-range mechanisms26,27.
Here we show that a native ear and an ear transplanted in tandem can overlappingly project to the same vestibular nuclei, suggesting an unknown guidance cue that aggregates those fibers preferentially in these nuclei. In contrast to the eye, the ear extracts information about movement and position in space using several distinct organs6,28. This feature of the ear allows to test whether similar or different sensory stimulation received by identical organs on two adjacent ears can affect the degree of overlap and segregation by simply putting the ears in line or off line with each during grafting. Three-eared frogs with normal ear orientation show normal swimming whereas a rotated third ear results in spinning comparable to the removal of one native ear. We show that two ears grafted in tandem with a similar orientation project largely in an overlapping manner within the vestibular nuclei. In contrast, ears with a 90° rotation with respect to each other result in predominantly segregated afferent projections within the vestibular nuclei. These data suggest that short-range mechanisms likely guide axons to the vestibular nucleus and their final position is likely fine-tuned by activity. Support of initial guidance being molecular comes from the observation of a distinct pattern of innervation of the mouse cochlear and vestibular nuclei prior to the onset of hearing and any known spontaneous activity10.
Results
Success of transplantation
Success for ear transplantation was defined as the detection of a third ear, whereas completeness of transplantation was defined as the amount and/or degree of normality of transplanted tissue. Of the 207 successful ear transplantations in which a third ear could be identified, 85% of the ears transplanted contained otoconia (n = 175/207 cases), whereas 14% were just a vesicle and lacked otoconia (n = 29/207 cases). Of the 175 transplanted ears that contained otoconia, 109 were nearly indistinguishable from a normal ear, showing two otoconia-bearing maculae and three semicircular canal cristae. In 52 transplantations, the transplanted ear fused with the native ear, forming a larger ear with multiple sets of otoconia. Fusion occurred more frequently with earlier transplantations (stage 25 vs stage 27). Transplanted ears were not detected in 17 animals. Only animals in which a complete third ear formed (Fig. 1), containing otoconia over the utricle and saccule and were either in the normal orientation (n = 38) or rotated by 90 degrees (n = 55), were used for further analysis.
Stage 46 Xenopus laevis ‘three-eared’ frogs.
(a) Embryo with a transplanted third ear in the native orientation. (b) Embryo with a transplanted ear rotated 90 degrees from the native ear. (a’) Higher magnification of the natively-oriented transplanted ear and the right native ear in A. (b’) Higher magnification of the 90 degree rotated transplanted ear and the right native ear in B. (c) Three-dimensional reconstruction of a 90 degree rotated transplanted ear next to the native ear. Endolymphatic space is magenta, endolymphatic duct is cyan and the hair cells are green. Native, unmanipulated ears are labeled ‘Ear’ and are circled with a black dotted line. Transplanted ears are indicated with a white arrow and are circled with a white dotted line. U, utricle; S, saccule. Blue and yellow arrows indicate ear orientation. Scale bar is 0.5 mm.
Ear transplantation in a rotated orientation perturbs normal vestibular function in tadpoles
Swimming behavior was used as a direct readout of vestibular function in tadpoles. Initial swimming behaviors, normal (swimming upright), swimming on side, swimming in a vertical orientation, swimming upside down and spinning were determined by observation for animals in which the third ear was in line with the native ear or rotated by 90 degrees (Table 1). Quantification of the duration of various behaviors observed in the first ten seconds of swimming showed that, for animals in which the transplanted ear was in the native orientation (Fig. 2b), most of the time spent swimming is in the upright orientation (Figs. 2b’ and 2e). Slightly less than half of tadpoles with an extra normally-oriented ear (45%) had one additional swimming behavior. Most commonly this was spinning, which was seen for a brief amount of time in 34% of these animals when they were first dropped into the arena. Spinning was immediately followed by re-orientation to upright swimming. This initial spinning behavior was also observed in 35% of control, unmanipulated animals (Fig. 2a), which also spent the majority of the time swimming upright (Fig. 2a’).
Swimming behavior of normal (two-eared), ‘three-eared’ and ‘one-eared’ frogs.
(a–d) Examples of embroys analyzed for their swimming behavior: (a) control, (b) normally-positioned third ear, (c) rotated third ear and (d) one-eared. (a’–d’) Quantification of percent time spent in various swimming orientations in the first ten seconds of recorded observation for each group of animals: (a’) control (n = 7), (b’)normally-positioned third ear (n = 12), (c’) rotated third ear (n = 11), (d’) one eared (n = 6). Behaviors other than upright swimming (blue) were pooled and referred to as “other behaviors” (orange). All comparisons are significant at p < 0.05 except where noted with horizontal bars and NS, not significant. Error bars reflect the standard error of the means. (e–h) Examples of swimming behaviors observed: (e) upright swimming, (f) upside down, (g) swimming on side, (h) spinning.
In animals in which the transplanted ear was rotated by 90 degrees (Fig. 2c), almost all animals were able to eventually swim upright (93%). Normal upright swimming was the first behavior observed in 31% of these animals. For those able to swim upright, all but two displayed additional swimming behaviors. Additional behaviors included one-sided swimming (49% of animals), upside-down swimming (22% of animals), vertical swimming (42% of animals) and spinning (42% of animals). Quantification of the duration of various behaviors observed in the first ten seconds after being dropped into water shows that animals, in which the transplanted ear was rotated by 90 degrees, spend 35.2 ± 8.9% of the time swimming in orientations other than upright (Figs. 2c’, 2f–2h), which is significantly more time spent than animals in which the transplanted ear was in the native orientation or than control animals [0.8 ± 0.6% and 1.8 ± 1.8%, respectively; p<0.05 (Figs. 2a–2c)]. Though the time spent in orientations other than upright swimming was less for animals in which the transplanted ear was rotated 90 degrees when compared with animals with only one ear (Figs. 2d and 2d’), the overall swimming behaviors were more similar. As with animals with a rotated transplanted ear, most animals with only one ear were able to eventually swim upright (85% of animals). Additional behaviors observed were one-sided swimming (60% of animals), upside-down swimming (25% of animals), vertical swimming (45% of animals) and spinning (90% of animals). Overall, these data show that asymmetrical vestibular input, either having an additional ear in a rotated position, or lacking an ear on one side, affects the swimming behavior of tadpoles whereas symmetric input through two or three similarly oriented ears does not.
Ear-orientation-dependent sorting of afferent projections to the Hindbrain
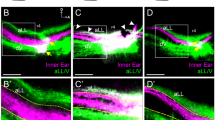
Lipophilic dyes of different fluorescence implanted into the native and transplanted ears of fourteen animals successfully labeled afferent projections in the hindbrain. The transplanted ear projected either with the native VIIIth ganglion, completely with its own ‘VIIIth’ ganglion, or in a mixture of both with the native and with their own ‘VIIIth’ ganglion (Fig. 3). In one animal, the transplanted ear, in the native orientation, projected entirely with the native VIIIth ganglion, although neurons from the two ears were mostly segregated within the ganglion (Fig. 3a). In seven animals, the transplanted ear projected with its own ‘VIIIth’ ganglion (Fig. 3b). Five of these animals had rotated ears; two had ears in the native orientation. In the remaining six animals, the transplanted ear partially overlapped with the native ganglion and partially was segregated (Figs. 3c and 3d). Two of these animals had rotated ears; four had ears in the native orientation. Axons from the additional ‘VIIIth’ ganglion from the transplanted ears entered the hindbrain at the approximate level of the trigeminal ganglion, though they did not terminate in the trigeminal nucleus.
Inner ear afferent projections.
(a) Animal in which inner ear afferents projected entirely together in the VIIIth ganglion. (b) Animal in which inner ear afferents from the natively-oriented transplanted ear projected in their own ‘VIIIth’ ganglion and entered the hindbrain separate from the inner ear afferents from the native VIIIth ganglion. (c) Animal in which the inner ear afferents from the 90 degrees rotated transplanted ear entered the hindbrain from both in its own ‘VIIIth’ ganglion and with the native VIIIth ganglion. (d) Animal in which the inner ear afferents leave the natively-oriented transplanted ear both in its own ‘VIIIth’ ganglion and along with the native VIIIth ganglion. Green arrowheads indicate projections from the transplanted ear. Red arrowheads indicate projections from the native ear. V, trigeminal nerve. Scale bar is 100 µm.
Afferents from all seven embryos with ears transplanted in line with the native ear projected to the same area in the hindbrain with nearly complete overlap of fibers from the two ears (Figs. 4a and 4a’). In contrast afferents from all seven embryos with ears transplanted 90 degrees offset from the native ear projected in general to the same area in the hindbrain, but show either a complete or nearly complete segregation of fibers from the two ears (Figs. 4b and 4b’). Projections into the hindbrain from the rotated ears were always located medial to the native ear projections (Figs. 4b and 4b’), but lateral to the trigeminal tract. For both transplants, in line and rotated, the vestibular nucleus innervated by two ears was broader than the vestibular nucleus innervated by only one ear (Fig. 4c) consistent with previous work showing shrinking after inner ear afferent removal29.
Overlap and segregation of inner ear afferents from transplanted and native ears.
(a-a’) Hindbrain from two animals in which the transplanted ear was in the native orientation showing overlap of sensory neurons from the native (red) and transplanted (green) ears. (b-b’) Hindbrain from two animals in which the transplanted ear was rotated by 90 degrees with respect to the native ear showing segregation of sensory neurons from the native (red) and transplanted (green) ears. Insets indicate the transplanted ear orientation. Red and green arrows indicate lipophilic dye placement. (a”) Intensity histogram from an animal with the transplanted ear (green) in line with the native ear (red) shows an overlap of intensity profiles in a single optical section. (b”) Intensity histogram from an animal with the transplanted ear (green) rotated by 90 degrees with respect to the native ear (red) shows a segregation of intensity profiles in a single optical section. (c) Hindbrain showing the native projection when only the control ear was on one side (red). (d) Mean percent overlap and standard error of sensory neurons from the native and transplanted ears for animals in which the transplanted ear was in line with or rotated by 90 degrees with respect to the native ear. 5 animals were analyzed for each condition. Each animal is the mean of measurements taken from 3 different optical sections. Error bars reflect the standard error of the means. ***, p<0.001. Scale bar is 25 µm.
The percent overlap of sensory neurons from the native and transplanted ears was calculated from intensity histograms obtained for the two sensory neuron populations (Figs. 4a”–4b”). When the transplanted ears were in line with the native ear, the percent overlap of the narrower histogram with the wider histogram was 97.7 ± 2.3% (n = 5) (Fig. 4d). The range of overlap for a single optical section in these animals was between 66–100%. When the transplanted ears were rotated by 90 degrees with respect to the native ear, the percent overlap of the narrower histogram with the wider histogram was 21.3 ± 6.4 (n = 5) (Fig. 4d). The difference in overlap profiles between animals with normally oriented transplanted ears compared with rotated transplanted ears was significant (p<0.0001). The range of overlap for a single optical section in these animals was between 0–57%. Overall, these data show that vestibular afferents are segregated in a manner dependent on the orientation and presumed differential sensory activity of the ear.
In summary, three eared frogs with normal tandem organization of native and transplanted ear showed near normal swimming behavior and overlapping projections of native and transplanted ear afferents. In contrast, three eared frogs with the transplanted ear rotated around 90 degrees relative to the native ear showed spinning behavior comparable to frogs with one ear removed and displayed near complete segregation of transplanted from native inner ear afferents. Data from mice showing afferent segregation prior to hair cell differentiation and even cell cycle exit of hair cells suggest that initial targeting by inner ear afferents is molecular and is only later fine-tuned through activity.
Discussion
The results presented here on ‘three-eared’ frogs show that inner ear vestibular afferents of transplanted ears project to the vestibular nucleus when they enter the hindbrain, partially or completely overlapping with the native inner ear projection. We also show different degrees of segregation of native and transplanted ear afferents within the vestibular nuclei depending on the orientation of the transplanted ear. Finally, the transplanted third ear affects swimming behavior of the tadpoles depending on the orientation.
The transplanted ear developed normally in 109 of 207 cases, supporting our previous data in frogs and data from chicks showing future sensory epithelia have already been specified by the otic placode stage30,31 and continue to differentiate normal after transplantation as previously described in various vertebrates32. The fusion of the transplanted ear and native ear may be a side-effect of the early transplantation, as this was observed less frequently when the ear was transplanted at stage 27 rather than at stage 25.
Using lipophilic dyes injected into the native ear and transplanted ear, we showed that, regardless of orientation, both ears project to the same vestibular nuclei of the hindbrain. That afferents from both ears project to and remain confined within the vestibular nuclei region suggests that initial guidance of inner ear sensory afferents is molecular-based, comparable to the targeting of retina ganglion cell (RGC) axons to the midbrain tectum1 or lateral line, inner ear and electroreceptive afferents in axolotl33. Work in mice has shown that cochlear afferents segregate from vestibular projections prior to the onset of hearing10, supporting the idea that initial targeting by inner ear afferents is through molecular guidance13,34,35,36. While the exact molecular nature of the guidance remains unknown, the Eph/Ephrin system used by RGCs to guide axons to the tectum/superior colliculus to maintain the visual field might apply to the ear afferents. Ephs and Ephrins have been shown to play some role in axon guidance in the auditory system35,36,37. Given that Ephs and Ephrins are also expressed in vestibular neurons38,39, suggests that Ephs and Ephrins may play a role in guiding sensory afferents to the proper location centrally in the vestibular nucleus, though this would need to be confirmed by further work.
While sensory neurons from the two ears projected to the same approximate hindbrain region, the precise targeting within the vestibular nucleus depends on the orientation of the transplanted ear with respect to the native ear. The nearly complete overlap in projections from the two ears when the transplanted ear was in the native orientation and the nearly complete segregation of projections from the two ears when the transplanted ear was rotated by 90 degrees suggest that activity-based mechanisms may play a role in fine tuning axon segregation in the vestibular system. The nearly complete segregation of sensory axons in the vestibular nucleus when the transplanted ear was rotated by 90 degrees resembled the ‘ocular dominance columns’ found in the visual system of mammals14,15 and experimentally induced in ‘three eyed frogs’16,40,41,42 as a result of differential activity between two eyes43,44,45,46,47,48,49. This activity-based segregation in the visual system likely is a compromise between the common molecular map of both eyes and the differential activity between the two eyes, resulting in a Hebbian sorting of afferents to distinct postsynaptic partners18. Extending this insight to the vestibular system, activity may help further segregate axons guided molecularly to vestibular nuclei due to different patterns of activation induced by head movement. Support of axon segregation comes from physiological recordings from second-order neurons in the vestibular nucleus. Recordings show activation by afferents from two different semicircular canals in only 10% of second-order neurons and activation by afferents from all three semicircular canals in only 2% of second-order neurons, whereas activation by afferents from a single semicircular canal occurs in 88% of recordings from second-order neurons12.
The ability of ‘three-eared’ frogs to swim normally depended upon the manipulation, which suggests that projections into the vestibular nucleus from the transplanted ear are functional. Animals in which the transplanted ear was in alignment with the native ear swam similar to control animals most of the time. In contrast, animals in which the transplanted ear was rotated by 90 degrees displayed more aberrant swimming, similar to that of animals with only one ear, though not as severe. Given that the latter two manipulations (rotated third ear or only one ear present) results in asymmetrical and mismatched gravitational sensation suggests that bilateral symmetry in sensory epithelia orientation is required for proper sensing of the animals' orientation to proper guide its swimming.
In conclusion, our data imply that mechanisms identified in retina projection development may operate in inner ear projection development: molecular cues and activity combine to wire the ear to vestibular nucleus neurons. Our data imply that evolution of sensory epithelia that acquire different sensory input, such as semicircular canals for angular acceleration perception28, or various auditory sensors50,51, will result in segregated projection allowing for segregated processing of unique information. Continued mutation and selection will eventually result in a segregated projection based on molecular cues52.
Methods
Ethics Statement
All methods were performed in accordance with the approved guidelines. All animal protocols used in these studies were approved by the Institutional Animal Care and Use Committee at the University of Iowa (#1303056).
Animals
Xenopus laevis embryos were obtained through induced ovulation using injection of human chorionic gonadotropin and fertilized with a sperm suspension in 1X Marc's Modified Ringer's Solution (MMR). Embryos were kept at 18°C in 90 mm Petri dishes containing 0.1X MMR (diluted from 1X MMR, see below) until they reached stage 4653.
Transplantations
Single otic placodes from donor X. laevis embryos were transplanted rostral to the native ear in host embryos at stages 25–27. Otic placodes were transplanted either in the native orientation or rotated by 90 degrees. Embryos were kept in 1X MMR for 10–15 min post-transplant to promote healing before being transferred to 0.1X MMR. Healing was confirmed visually as a fusion of the ectoderm above the transplant. Transplants were monitored daily for continued growth. Normal development of multiple otoconia bearing organs of transplanted ear formation was considered to indicate success of transplantations. Only animals with a completely formed transplanted ear, with recognizable features to indicate the orientation of the ear, such as otoconia overlaying the utricle and saccule, were used for further analysis. After embryos reached stage 46, their swimming behavior was assessed (see below). Subsequently animals were anesthetized in 0.02% Benzocaine and fixed in 10% PFA by immersion.
Swimming Behavior
Animals were dropped into a 3”× 4” swimming arena to observe their initial startle and swimming behavior. All initial behaviors were scored as the following: normal (swimming upright), swimming on side, swimming upside down, swimming vertical and spinning. Personal observations of all behaviors were recorded for approximately one minute, or until the animal ceased swimming. A sample of animals from each condition were selected and the first ten seconds after they were dropped into the arena were recorded. Percentage of time was calculated for upright swimming and for the other behaviors pooled. Data is reported as means and standard errors of the means. Significance was determined using a two-way ANOVA (Vassar Stats) and post-hoc test (GraphPad). Images for Figure 2 were captured with a Canon T2i camera set at burst recording mode.
Lipophilic dye labelling
Small pieces of dye-soaked filter paper54,55 were flattened and implanted into the native (NeuroVue™ Maroon) and transplanted ears (NeuroVue™ Red) in animals previously fixed in 10% PFA. Animals were kept at 36°C for approximately 18 hours to allow for dye diffusion into the hindbrain. Filter paper was removed prior to imaging. Brains were mounted ventral-side up on a slide in glycerol and imaged with a Leica TCS SP5 confocal microscope with a 40x water immersion objective.
Quantification of sensory neuron overlap
The percent overlap of sensory afferents in the vestibular nucleus of ‘three-eared’ frogs when the transplanted ear was either in the native orientation or rotated by 90 degrees was calculated using the Leica software. Three optical sections were selected for each animal, near the top, middle and bottom of the image stack. A region of interest line was drawn perpendicularly through the sensory afferents at the approximate midpoint rostral- caudal of the descending tract of the vestibular nucleus. The descending tract of the vestibular nucleus was defined as being outlined by those afferents that projected caudally upon entering the hindbrain. Using the line profile intensity tool, an intensity histogram was provided for the channels representing the two populations of sensory neurons, belonging to the native and transplanted ear. The medial and lateral boundaries of the two histograms were determined manually by using the boundaries generated from a threshold omitting the bottom 25% of the relative intensity. Animals in which either of the fluorescent signals was weak, making it difficult to generate an accurate intensity profile, were not included in the analysis. The percent overlap was calculated by determining the percent of the narrower histogram contained within the wider histogram. The percent overlap obtained for each of the three optical sections was pooled to provide a percent overlap per animal. Animals were pooled to provide the mean percent overlap for the two orientations of ‘three-eared’ frogs. The means and standard errors were calculated. Student's t-test was used as a test for significance.
References
Triplett, J. W. Molecular guidance of retinotopic map development in the midbrain. Curr Opin Neurobiol 24, 7–12 (2014).
Adam, Y. & Mizrahi, A. Circuit formation and maintenance—perspectives from the mammalian olfactory bulb. Curr Opin Neurobiol 20, 134–140, 10.1016/j.conb.2009.11.001 (2010).
Gliem, S. et al. Bimodal processing of olfactory information in an amphibian nose: odor responses segregate into a medial and a lateral stream. Cell Mol Life Sci 70, 1965–1984, 10.1007/s00018-012-1226-8 (2013).
Cheetham, C. E. & Belluscio, L. An Olfactory Critical Period. Science 344, 157–158, 10.1126/science.1253136 (2014).
Duncan, J. S. & Fritzsch, B. Continued expression of GATA3 is necessary for cochlear neurosensory development. PLoS One 8, e62046, 10.1371/journal.pone (2013).
Straka, H., Fritzsch, B. & Glover, J. C. Connecting Ears to Eye Muscles: Evolution of a ‘Simple’ Reflex Arc. Brain, Behav Evol 83, 1–14 (2014).
Jahan, I., Kersigo, J., Pan, N. & Fritzsch, B. Neurod1 regulates survival and formation of connections in mouse ear and brain. Cell Tissue Res 341, 95–110, 10.1007/s00441-010-0984-6 (2010).
Clause, A. et al. The precise temporal pattern of prehearing spontaneous activity is necessary for tonotopic map refinement. Neruon 82, 822–835 (2014).
Maklad, A. & Fritzsch, B. Development of vestibular afferent projections into the hindbrain and their central targets. Brain res bull 60, 497–510 (2003).
Fritzsch, B., Pan, N., Jahan, I. & Elliott, K. Inner ear development: building a spiral ganglion and an organ of Corti out of unspecified ectoderm. Cell Tissue Res, 1–18, 10.1007/s00441-014-2031-5 (2015).
Maklad, A., Kamel, S., Wong, E. & Fritzsch, B. Development and organization of polarity-specific segregation of primary vestibular afferent fibers in mice. Cell Tissue Res 340, 303–321, 10.1007/s00441-010-0944-1 (2010).
Straka, H., Biesdorf, S. & Dieringer, N. Canal-Specific Excitation and Inhibition of Frog Second-Order Vestibular Neurons. J Neurophysiol Vol. 78, 1363–72 (1997).
Maklad, A. & Fritzsch, B. The developmental segregation of posterior crista and saccular vestibular fibers in mice: a carbocyanine tracer study using confocal microscopy. Dev Brain Res 135, 1–17, http://dx.doi.org/10.1016/S0165-3806(01)00327-3 (2002).
Hubel, D. H. & Wiesel, T. N. Functional architecture of macaque monkey visual cortex. Proc R Soc London B 198, 1–59 (1977).
Wiesel, T. N. The postnatal development of the visual cortex and the influence of environment. Biosci Rep 2, 351–377, 10.1007/bf01119299 (1982).
Constantine-Paton, M. & Law, M. Eye-specific termination bands in tecta of three-eyed frogs. Science 202, 639–641, 10.1126/science.309179 (1978).
Shatz, C. J. et al. Pioneer neurons and target selection in cerebral cortical development. Cold Spring Harb Symp Quant Biol 55, 469–480 (1990).
Kirkby, L. A., Sack, G. S., Firl, A. & Feller, M. B. A role for correlated spontaneous activity in the assembly of neural circuits. Neuron 80, 1129–1144, 10.1016/j.neuron.2013.10.030 (2013).
Schmidt, J. T. & Tieman, S. B. Eye-specific segregation of optic afferents in mammals, fish and frogs: the role of activity. Cell Mol Neurobiol 5, 5–34 (1985).
Morrison, E. & Graziadei, P. C. An ultrastructural study of glomeruli associated with vomeronasal organs transplanted into the rat CNS. Anat Embryol 193, 331–339, 10.1007/bf00186690 (1996).
Graziadei, P. P., Levine, R. R. & Graziadei, G. A. Regeneration of olfactory axons and synapse formation in the forebrain after bulbectomy in neonatal mice. Proc Natl Acad Sci 75, 5230–5234 (1978).
Graziadei, P. P. C., Levine, R. R. & Monti Graziadei, G. A. Plasticity of connections of the olfactory sensory neuron: Regeneration into the forebrain following bulbectomy in the neonatal mouse. Neurosci 4, 713–727, 10.1016/0306-4522(79)90002-2 (1979).
Graziadei, P. P. C. & Samanen, D. W. Ectopic glomerular structures in the olfactory bulb of neonatal and adult mice. Brain Res 187, 467–472 (1980).
Magrassi, L. & Graziadei, P. P. C. Interaction of the transplanted olfactory placode with the optic stalk and the diencephalon in Xenopus laevis embryos. Neurosci 15, 903–921, 10.1016/0306-4522(85)90088-0 (1985).
Blackiston, D. J. & Levin, M. Ectopic eyes outside the head in Xenopus tadpoles provide sensory data for light-mediated learning. J Exp Biol 216, 1031–1040, 10.1242/jeb.074963 (2013).
Elliott, K. L., Houston, D. W. & Fritzsch, B. Transplantation of Xenopus laevis Tissues to Determine the Ability of Motor Neurons to Acquire a Novel Target. PLoS One 8, e55541 (2013).
Leber, S. M. & Model, P. G. Effect of precocious and delayed afferent arrival on synapse localization on the amphibian Mauthner cell. J Comp Neurol 313, 31–44, 10.1002/cne.903130104 (1991).
Fritzsch, B. & Straka, H. Evolution of vertebrate mechanosensory hair cells and inner ears: toward identifying stimuli that select mutation driven altered morphologies. J Comp Physiol A 200, 5–18, 10.1007/s00359-013-0865-z (2014).
Fritzsch, B. Experimental reorganization in the alar plate of the clawed toad, Xenopus laevis. I. Quantitative and qualitative effects of embryonic otocyst extirpation. Dev Brain Res 51, 113–122 (1990).
Elliott, K. L. & Fritzsch, B. Transplantation of Xenopus laevis ears reveals the ability to form afferent and efferent connections with the spinal cord. Int J Dev Biol 54, 1443–1451, 10.1387/ijdb.103061ke (2010).
Abelló, G. et al. Independent regulation of Sox3 and Lmx1b by FGF and BMP signaling influences the neurogenic and non-neurogenic domains in the chick otic placode. Dev Biol 339, 166–178, 10.1016/j.ydbio.2009.12.027 (2010).
Fritzsch, B., Barald, K. & Lomax, M. in Springer Handbook of Auditory Research. Vol XII. Development of the Auditory System. (eds E. W. Rubel, A. N. Popper, & R, R. Fay ) 80–145 (Springer Verlag, New York, 1998).
Fritzsch, B., Gregory, D. & Rosa-Molinar, E. The development of the hindbrain afferent projections in the axolotl: evidence for timing as a specific mechanism of afferent fiber sorting. Zoology 108, 297–306 (2005).
Maklad, A. & Fritzsch, B. Development of vestibular afferent projections into the hindbrain and their central targets. Brain Res Bull 60, 497–510 (2003).
Allen-Sharpley, M. R. & Cramer, K. S. Coordinated Eph-ephrin signaling guides migration and axon targeting in the avian auditory system. Neural Dev 7, 29 (2012).
Allen-Sharpley, M. R., Tjia, M. & Cramer, K. S. Differential Roles for EphA and EphB Signaling in Segregation and Patterning of Central Vestibulocochlear Nerve Projections. PLoS One 8, e78658 (2013).
Defourny, J. et al. Ephrin-A5/EphA4 signalling controls specific afferent targeting to cochlear hair cells. Nat Commun 4, 1438 (2013).
Bianchi, L. M. & Liu, H. Comparison of Ephrin-A ligand and EphA receptor distribution in the developing inner ear. The Anatomical Record 254, 127–134, 10.1002/(sici)1097-0185(19990101)254:1127::aid-ar16>3.0.co;2-q (1999).
Cowan, C. A., Yokoyama, N., Bianchi, L. M., Henkemeyer, M. & Fritzsch, B. EphB2 guides axons at the midline and is necessary for normal vestibular function. Neuron 26, 417–430 (2000).
Constantine-Paton, M. in Developmental Order: Its Origin and Regulation (ed S. Subtelny ) 317–349 (Alan R. Liss, New York, 1982).
Springer, A. D. & Cohen, S. M. Optic fiber segregation in goldfish with two eyes innervating one tectal lobe. Brain Res 225, 23–36, 10.1016/0006-8993(81)90315-2 (1981).
Easter, S. S. Postnatal neurogenesis and changing connections. Trends Neurosci 6, 53–56, 10.1016/0166-2236(83)90025-5 (1983).
Hubel, D. H. & Wiesel, T. N. Binocular interaction in striate cortex of kittens reared with artifical squint. J Neurophysiol 28, 1041–1059 (1965).
Swindale, N. V. Absence of ocular dominance patches in dark reared cats. Nature 290, 332–333 (1981).
Swindale, N. V. & Cynader, M. S. Physiological segregation of geniculocortocal afferents in the visual cortex of dark reared cats. Neurosci Abstr 9, 24 (1983).
Meyer, R. Tetrodotoxin blocks the formation of ocular dominance columns in goldfish. Science 218, 589–591, 10.1126/science.7123262 (1982).
Mower, G. D., Christen, W. G. & Caplan, C. J. Absence of ocular dominance columns in binocularly deprived cats. Investig Opthalmol Vis Sci 25 (Suppl.), 214 (ARVO abstr.) (1984).
Boss, V. & Schmidt, J. Activity and the formation of ocular dominance patches in dually innervated tectum of goldfish. J Neurosci 4, 2891–2905 (1984).
Reh, T. & Constantine-Paton, M. Eye-specific segregation requires neural activity in three-eyed Rana pipiens. J Neurosci 5, 1132–1143 (1985).
Fritzsch, B., Niemann, U. & Bleckmann, H. A discrete projection of the sacculus and lagena to a distinct brainstem nucleus in a catfish. Neurosci Lett 111, 7–11, 0304-3940(90)90335-7 (1990).
Fritzsch, B. et al. Evolution and development of the tetrapod auditory system: an organ of Corti-centric perspective. Evol Dev 15, 63–79, 10.1111/ede.12015 (2013).
Fritzsch, B., Pauley, S., Feng, F., Matei, V. & Nichols, D. H. The molecular and developmental basis of the evolution of the vertebrate auditory system. Int J Comp Psychol 19, 1–24 (2006).
Nieuwkoop, P. & Faber, J. Normal Table of Xenoups laevis (Daudin) (Garland Publishing, INC, New York, 1994).
Fritzsch, B., Muirhead, K. A., Feng, F., Gray, B. D. & Ohlsson-Wilhelm, B. M. Diffusion and imaging properties of three new lipophilic tracers, NeuroVue(TM) Maroon, NeuroVue(TM) Red and NeuroVue(TM) Green and their use for double and triple labeling of neuronal profile. Brain Res Bull 66, 249–258 (2005).
Tonniges, J. et al. Photo- and bio-physical characterization of novel violet and near-infrared lipophilic fluorophores for neuronal tracing. J Microsc 239, 117–134 (2010).
Acknowledgements
This work was in part supported by NASA under Grant No. NNX10AK63H and in part by NIH under Grant Nos. R01 DC055095590 (B.F.), P30 DC010362 (University of Iowa) and R01 GM083999 (D.W.H.). We would like to thank Dr. Michael Dailey for his assistance with the Leica software used for the quantification of sensory afferent overlap and Dr. M. Constantine-Paton for helpful suggestions on the manuscript. The use of the Leica TCS SP5 multi-photon confocal microscope was made possible by a grant from the Roy. J. Carver Charitable Trust. We also thank the Office of the Vice President for Research (OVPR) of the University of Iowa for support.
Author information
Authors and Affiliations
Contributions
K.L.E. wrote the manuscript, D.W.H. and B.F. edited the manuscript. K.L.E. and B.F. designed the experiment, D.W.H. provided significant feedback to the design and interpretation of the data. All authors reviewed the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Elliott, K., Houston, D. & Fritzsch, B. Sensory afferent segregation in three-eared frogs resemble the dominance columns observed in three-eyed frogs. Sci Rep 5, 8338 (2015). https://doi.org/10.1038/srep08338
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep08338
This article is cited by
-
Topologically correct central projections of tetrapod inner ear afferents require Fzd3
Scientific Reports (2019)
-
A New Model for Congenital Vestibular Disorders
Journal of the Association for Research in Otolaryngology (2019)
-
Ear transplantations reveal conservation of inner ear afferent pathfinding cues
Scientific Reports (2018)
-
Serotonergic stimulation induces nerve growth and promotes visual learning via posterior eye grafts in a vertebrate model of induced sensory plasticity
npj Regenerative Medicine (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.