Abstract
Until now, it has been well-established that coral complex in the Arabian/Persian Gulf only exist in the coastal regions of Bahrain, Iran, Kuwait, Oman, Qatar, Saudi Arabia and United Arab Emirates and it was thought that there are no coral reefs in Iraq. However, here for the first time we show the existence of a living 28 km2 large coral reef in this country. These corals are adapted to one of the most extreme coral-bearing environments on earth: the seawater temperature in this area ranges between 14 and 34°C. The discovery of the unique coral reef oasis in the turbid coastal waters of Iraq will stimulate the interest of governmental agencies, environmental organizations, as well as of the international scientific community working on the fundamental understanding of coral marine ecosystems and global climate today.
Similar content being viewed by others
Introduction
A narrow strip (58 km) of the northern coast of the Arabian/Persian Gulf belongs to Iraq1. The area is dominated by the large swampy river delta of the rivers Euphrates, Tigris and Karun, merging into the Shatt al-Arab that represents the main outflow in the Arabian/Persian Gulf. Its water is not only sediment-loaded but it is also often oil-polluted. The sediment particles are distributed throughout the Gulf by strong winds and currents generated during winter. The seasonally varying turbidity near the delta and along part of the Iranian coast is the reason for the hypothesized absence of coral reefs in this region2. Furthermore, it is accepted that the Coriolis effect deflects the Shatt al-Arab river plume towards Kuwait and suppresses reef growth there. Reefs along the Kuwait mainland are only found at Ras az Zaur in southern Kuwait and on the southern offshore islands (Kubbar, Qaro and Umm Al-Maradim)3. The last of Kuwait's coastal fringing reefs, which probably did extend in the recent past as far north as Ra's al-Ardh, is currently under threat from large-scale urban and industrial development4.
The Shatt al-Arab, the only continuous freshwater source in the region, causes salinities to decrease in the extreme north to about 36‰ in summer5. Underwater visibility is often limited to 1 m or less and at a depth of 1 m, turbidity may reach 0.7 NTU6,7. Therefore, the turbid waters of Iraq coastal territory prevented the detection of the potential presence of coral reefs in this area, on satellite observations8. Joint expeditions performed by scientific scuba divers from MSC Basrah (Iraq) and SDC Freiberg (Germany) carried out in September 2012 and in May 2013, revealed the existence of a true live coral reef in Iraqi coastal waters for the first time ever (Fig. 1; Supplementary Information - Video). This newly discovered coral reef differs from counterparts established in adjacent Kuwait. In fact, the reefs in Kuwait are fringing reefs distributed around islands9, or close to the coast at water depths between 0–10 m. These reefs are settled on sandy ground in relatively transparent water. The newly discovered reef at the mouth of the Shatt al-Arab is located at greater depths in a zone of low visibility and rapidly changing conditions (temperature and salinity) due to strong currents. These currents are triggered by tides and the significant freshwater sediment-loaded discharge by the Shatt el-Arab (44.8 × 109 m3/a and 2700 kg/m3)10.
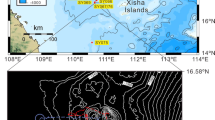
A reef complex in Iraqi waters discovered for the first time.
(a) Region map. Base map provided by ReefBase (http://www.reefbase.org). (b) Detailed depths map of Palinurus rock reef. (c) Stony coral Platygyra pini Chevalier, showing anchor damages (width of the image: ca. 1 m). (d) Octocoral Menella sp. Gray with different Ophiotheia venusta brittle stars (width of the image: ca. 30 cm). The coloration of the living specimen is as follows: the dorsal disc is red in its center and marbled with white, yellow and black towards its perimeter. The dorsal surface of the arms is cross-banded with yellow, white and black34. (Underwater images were made by Thomas Pohl and maps were made by Sameh W. Al-Muqdadi).
Results
The coral reef has an area of 28 km2 and is located at 29°37′00 N and 048°48′00 E (Fig. 1b). The primary survey identified a 6 by 3 km wide zone of relatively healthy reefs at water depths between 7 and 20 m. The site is characterized by a tidal variation of about 3 m, rather strong tidal currents (3–4.5 m/s), high turbidity (see video in Supplementary Information). There is a high nutrient load from rivers and thus dim light in consequence.
A number of living stone corals like Platygyra pini (Chevalier, 1975) (Fig. 1c), Turbinaria stellata (Lamark, 1816), Tubastrea sp., Porites lobata (Dana, 1846), Porites sp., Astroides calycularis (Pallas, 1766), Goniastrea edwardsi (Chevalier, 1971) as well as octocorals Junceella juncea (Pallas, 1766) with several ophiuroids (Fig. 1d) were identified from the Iraq coral reef. Ophiuroidea (Phylum Echinodermata) like brittle stars, serpent stars and basket stars11 are still very poorly investigated in the Arabian/Persian Gulf. The brittle stars we observed on the branches of gorgonians are Ophiotheia venusta (Fig. 1d). These were invariably entwined around the branches of octocorals like Menella sp.12 when we recoded them in deeper parts of the reef. Aside from the Danish scientific expedition in 194013, there are only a few recent reports on ophiuroids. All these are related to those found in Iranian coastal waters14, such as Ophiocoma erinaceus (Clark & Rowe, 1971).
We also observed sponges and bivalves that may compete with corals for space on the reef, or that may bio-erode the coral structure and therefore can damage or destroy coral colonies. For example, we revealed living filter-feeding bivalves (Lithophaga robusta), which possess a high bio-eroding activity, within stony corals (Fig. 2b).
(a) Isolated fragment of the coral Platygyra pini with evidence of the presence of filter-feeding bio-eroding bivalves (Lithophaga robusta) (b). The greenish colour of this coral species (c) is assumed to be due to microalgal symbionts in spite of the fact that this animal lives in turbid and shallow waters. (Images were made by Hermann Ehrlich).
The poor knowledge of sponges (Porifera) from the region has been reported previously15,16. To identify sponges in this marine environment was entirely surprising, as it was suggested previously17 that the sensitivity of sponges to suspended sediment may exclude them from high-sediment habitats. However, we suggest that the abundance of silica-containing demo-sponges in the discovered coral reef can be caused by silicate-rich waters: the highest values (ca. 6 μmol/kg) were measured in the outflow of the Shatt al-Arab18. There is neither a comprehensive study on the sponge fauna of the northern part of the Persian Gulf in general, nor one on the Palinurus Rock region. Therefore, the discovery of several representatives of Porifera, including green collared encrusting sponge (Fig. 3) is of crucial importance for both ecology and biodiversity of this very unusual coral reef system. We suggest that this sponge also harbours photosynthetic endosymbionts (cyanobacteria, dinoflagellates) in a manner comparable to that of corals. Preliminary analysis of external shape and spicules morphology19 showed the presence of 5 species of at least 3 different genera of demosponges in the studied area, including Haliclona sp. Similar sponges has been reported previously near Larak Island, Iran16.
Discussion
We were entirely surprised to find living coral reef under such harsh conditions. Our discovery will yield critical intelligence about the way these rare systems respond to both human and naturally occurring environmental changes.
Previously, coral reef development in turbid water as it is the case here described was the subject of numerous studies (for a review see20,21,22,23). Usually, extensive coral reefs do not typically develop under conditions where nutrient and suspended sediment concentrations are acutely or chronically very high24. Turbidity over coral reef systems is mostly controlled by sediment (both organic and inorganic) resuspension and/or transport from terrestrial systems25. Differential species response to similar sediment loadings is known: large colonies, and/or species with thick tissues, are often relatively resistant. Meanwhile, smaller colonies and species with thin tissue layers may be greatly affected23. As recently reviewed by Risk & Edinger23, a consequence of reduced recruitment in heavily sedimented reefs is that the population structure of the surviving coral will shift toward a preponderance of large, old colonies. Furthermore, massive coral colonies possess multilobate morphology, which allows the coral colony to continue extending upward in some portions, while other portions of the colony grow slowly. It remains unclear to what extent biodiversity is related to the resilience and robustness of such unusual coral reefs like those in the Arabian/Persian waters5. However, four of coral taxa (Platygyra, Porites, Goniastrea and Turbinaria) observed by us are slow-growing massive species. They are related to one of the main clade, the “robust” corals26 with relatively solid, heavily calcified skeletons that result from the solid construction of corallite walls. Two other observed taxa, Tubastrea and Astroides are related to Dendrophylliidae family that include typical representatives of the “complex” clade. Interestingly, Tubastrea species are known as azooxanthellate scleractinian corals with high resistance to pollution, for example with respect to metals27. Astroides calycularis has been described as a species living in both light and dark28 that habituates localities with strong water movement29. We suggest that representatives of both clades can serve as bioindicators for the highly changeable conditions in the Shatt al Arab area.
It cannot be excluded that this coral reef system possess specific metabolism.
For example, some Indo- Pacific reefs flourish at Suspended Particulate Matter levels as high as 10 mg/L, in some cases by shifting their metabolism toward more heterotrophy in murky waters30. Probably, such coral reef communities can take up suspended planktonic organic matter (detritus, phytoplankton, zooplankton) as a source of carbon they do not fix themselves via photosynthesis31. This kind of suspended organic matter seems to be unimportant as a source of carbon for many hard and soft coral communities. However, it is an important source of specific essential nutrients for many communities and food for some coral reef associated organisms like gorgonians and sponges. The contribution of sponges and their associations with other organisms under high sedimentation conditions in the coral reef we have discovered remain unknown.
The temperature on earth is increasing and extremes are becoming more pronounced. Study of these extreme reef systems becomes of vital relevance as our climate changes. The large temperature variations in these Arabian/Persian waters has been recently described as an ideal biomarker by which coral reef persistence under changing global conditions may be evaluated5,32.
This area in Iraq has witnessed extensive historical impacts from a number of significant environmental, climatic and human stressors. Further investigations are urged to monitor and catalogue the biodiversity within this unique turbid water tolerant coral reef community.
According to the WWF, several factors inhibit international collaboration to perverse such globally important resources. Among these are military conflicts, the lack of adequate coordination and sufficient management capacity, as well as principal disharmony among littoral states. These factors endanger conservation and the survival of these valuable marine ecosystems33.
These valuable habitats urgently need protection, conservation and research. This is a particular challenge the Gulf area due to the extensive oil and gas exploration. Countries formerly experiencing major disputes now share common marine habitats, opening the door for political as well as scientific action. Monitoring these rare reefs will require intense communication between experts from many nations and the results will have global implications.
Methods
Exploration of the reef complex was performed by experienced scientific divers from the SDC of the TU Bergakademie Freiberg. Kind support was provided from scientific divers from MSC of the University of Basrah, using the research vessel “Al-Bahith” at several locations of the reef complex. Samples were taken in plastic boxes and transported to the surface. All samples were immediately preserved in methanol for further investigations and documentation in the laboratory. Taxonomic designation of sponges was done based on scanning optical microscopy, skeletal slides, dissociated spicule mounts and tissue samples. Water temperature and depths were read from the diving computer. Coordinates were taken with a GARMIN handheld GPS. Depths were measured by professional diving computer Suunto D9 using function of Altitude and maximum depth display. Figures and maps were prepared using GNU Image Manipulation Program "GIMP 2.8".
References
Bird, E. C. F. Encyclopedia of the World's Coastal Landforms. Band 1. (Springer, Heidelberg, 2010).
Emery, K. O. Sediments and water of the Persian Gulf. Bull AAPG 401, 2354–2383 (1956).
Gischler, E. & Lomando, A. J. Offshore sedimentary facies of a modern carbonate ramp, Kuwait, northwestern Arabian-Persian Gulf. Facies 50, 443–462 (2005).
Levin, S. A. & Lubchenco, J. Resilience, robustness and marine ecosystem-based management. BioScience 58, 27–32 (2008).
Riegl, B. M. & Purkis, S. J. Coral Reefs of the World: Adaptation to Climatic Extremes. 3, 379 (Springer, Dordrecht-Heidelberg-New York-London, 2012).
Downing, N. Coral communities in an extreme environment: The northwestern Arabian Gulf. Proc. 5th Int. Coral Reef Congress, Tahiti 6, 343–348 (1985).
Hodgson, G. & Carpenter, K. Scleractinian Corals of Kuwait. Pacific Science 49, 227–246 (1995).
Rezai, H., Wilson, S., Claereboudt, M. & Riegl, B. M. Coral Reef Status in the Ropme Sea Area: Arabian/Persian Gulf, Gulf Of Oman and Arabian Sea. In: Status of Coral Reefs of the World (ed. Wilkinson, C.), 1, 301 (Australian Institute of Marine Science, Australia, Queensland, Townsville, 2004).
Sheppard, C. R., Wilson, S. C., Salm, R. V. & Dixon, D. Reefs and coral communities of the Arabian Gulf and Arabian Sea. In: Coral Reefs of the Indian Ocean: Their Ecology and Conservation. (eds. McClanahan, T. R., Sheppard, C. R. & Obura, D.), 257–294 (Oxford University Press, Oxford, 2000).
Hovius, N. Controls on sediment supply by large rivers. In: Relative Role of Eustasy, Climate and Tectonism in Continental Rocks. (eds. Shanley, K. W. & McCabe, P. J.), 59, 3–16 (SEPM Publications, U.S., Tulsa, 1998).
Coleman, N. Sea Stars: Echinoderms of Asia/Indo-Pacific. (Neville Coleman's Underwater Geographic Pty Ltd, Australia, 2007).
George, D. J. Reef-Associated Macroinvertebrates of the SE Gulf. In: Coral Reefs of the World: Adaptation to Climatic Extremes (eds. Riegl, B. M. & Purkis, S. J.) 3, 379 (Springer, Dordrecht-Heidelberg-New York-London, 2012).
Keshavarz, M., Mohammadikia, D. & Dabbagh, A. R. The Echinoderms Fauna in Intertidal zone of Southern Oli village coast (Boushehr, Persian Gulf). J. Anim. Sci. Adv. 2, 495–498 (2012).
Fatemi, S. M. R., Jamili, S., Valinassab, T. & Kuranlu, N. Diversity of Ophiuroidea from Lengeh Portand Qeshm Island in the Persian Gulf. J. Fish. Aqua. Sci. 5, 42–48 (2010).
van Soest, R. W. M. & Beglinger, E. J. Tetractinellid and hadromerid sponges of the Sultanate of Oman. Zool. Med. Leiden 82, 749–790 (2008).
Khoshkhoo, Zh. et al. First record of siliceous and calcareous sponges from Larak Island, Persian Gulf – Iran. Middle-East J. Sci. Res. 11, 887–893 (2012).
Gerrodette, T. & Flechsig, A. O. Sediment-induced reduction in the pumping rate of the tropical sponge Verongia lacunosa. Mar. Biol. 55, 103–110 (1979).
Brewer, P. G. & Dyrssen, D. Chemical oceanography of the Persian Gulf. Prog. Oceanography 14, 41–55 (1985).
Hooper, J. N. A. Guide to Sponge Collection and Identification. (Queensland Museum, Australia, South Brisbane, 2000).
Roy, K. J. & Smith, S. V. Sedimentation and coral reef development in turbid water: Fanning Lagoon. Pac. Sci. 25, 234–248 (1971).
Woolfe, K. J. & Larcombe, P. Terrigenous sedimentation and coral reef growth: a conceptual framework. Mar. Geol. 155, 331–345 (1998).
Ogston, A. S., Storlazzi, C. D., Field, M. E. & Presto, M. K. Sediment resuspension and transport patterns on a fringing reef flat, Molokai, Hawaii. Coral Reefs 23, 559–569 (2004).
Risk, M. J. & Edinger, E. Impact of sediment on coral reef. In: Encyclopedia of Modern Coral Reefs: Structure, Form and Process (ed. Hopley, D.), 575–586, (Springer Science + Business Media B.V., 2011).
Furnas, M. & Mitchell, A. Runoff of terrestrial sediment and nutrients into the Great Barrier Reef World Heritage Area. In: Oceanographic Processes of Coral Reefs: Physical and Biological Links in the Great Barrier Reef. (ed. Wolanski, E.), 37–52, (CRC Press, USA, Boca Raton, 2000).
Otero, E. & Carbery, K. K. Chlorophyll a and turbidity patterns over coral reefs systems of La Parguera Natural Reserve, Puerto Rico. Rev. Biol. Trop. (Int. J. Trop. Biol.) 53 (Suppl. 1), 25–32 (2005).
Kitahara, M. V., Cairns, S. D., Stolarski, J., Blair, D. & Miller, D. J. A Comprehensive Phylogenetic Analysis of the Scleractinia (Cnidaria, Anthozoa) Based on Mitochondrial CO1 Sequence Data. PLoS ONE 5, e11490; 10.1371/journal.pone.0011490 (2010).
Chan, I., et al. Comparison of metal accumulation in the azooxanthellate scleractinian coral (Tubastraea coccinea) from different polluted environments. Mar Pollut Bull. 10.1016/j.marpolbul.2013.11.015 (2013, available online 7 December 2013, in press, corrected proof).
Grubelic, I., Antolic, B., Despalatovi, M., Grbec, B. & Paklar, B. G. Effect of climatic fluctuations on the distribution of warm-water coral Astroides calcyularis in the Adriatic Sea: new records and review. J. Mar. Biol. Assoc. UK 84, 599–602 (2004).
Casado-Amezua, P., Gasparini, G. & Goffredo, S. Phenological and morphological variations in the Mediterranean orange coral Astroides calycularis between two distant localities. Zoology 116, 159–167 (2013).
Anthony, K. R. N. Enhanced particle-feeding capacity of corals on turbid reefs (Great Barrier Reef, Australia). Coral Reefs 19, 59–67 (2000).
Atkinson, M. J. Carbon fluxes of coral reefs. In: Encyclopedia of Modern Coral Reefs. (ed. Hopley, D.), 181–185, (Springer Science + Business Media B.V. 2011).
Riegl, B. Global climate change and coral reefs: different effects in two high latitude areas (Arabian Gulf, South Africa). Coral Reefs 22, 433–446 (2003).
Al Cibahy, A., et al. Coral reef conservation in Abu Dhabi and the United Arab Emirates. Reef Encounter 36, 14–15 (2008).
Gibbs, P. E., Clark, A. M. & Clark, C. M. Echinoderms from the northern region of the Great Barrier Reef, Australia. Bull. Br. Mus. nat. Hist. (Zool.) 30, 103–144 (1976).
Acknowledgements
German scientist from TU Bergakademie Freiberg received financial support through the DAAD (German Academic Exchange Service) financed project GRI (Geoscience Resources Iraq). The MSC of the University of Basrah provided logistic support, diving equipment and the research vessel Al-Bahith. We express our deep thanks to the crew of Al-Bahith for their enthusiastic help and enduring support. This research was partially as well supported by DFG Grant EH 394/3-1 and BHMZ Programme of Dr.-Erich-Krüger- Foundation (Germany). We cordially thanks Dr. Allison L. Stelling for editing of the manuscript.
Author information
Authors and Affiliations
Contributions
The marine expedition plan was designed by T.P., M.H.A. and B.M. The field sampling programme was orchestrated and implemented by T.P., S.W.A.-M., F.N.A.-M. and B.M. Data analysis and interpretation was conducted by H.E., T.P. and S.W.A.-M. The manuscript was written by H.E., T.P. and B.M. Underwater images, maps and video were made by T.P. and S.W.A.-M. Photographs of isolated coral fragments were made by H.E.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary video
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Pohl, T., Al-Muqdadi, S., Ali, M. et al. Discovery of a living coral reef in the coastal waters of Iraq. Sci Rep 4, 4250 (2014). https://doi.org/10.1038/srep04250
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep04250
This article is cited by
-
Pseudanuretes anfoozi n. sp. (Copepoda: Siphonostomatoida: Caligidae) from the yellowbar angelfish Pomacanthus maculosus (Forsskål) in coral reefs off Iraq
Systematic Parasitology (2020)
-
Modeling abundance, growth, and health of the solitary coral Scolymia wellsi (Mussidae) in turbid SW Atlantic coral reefs
Marine Biology (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.