Abstract
Transparent inorganic luminescent materials have attracted considerable scientific and industrial attention recently because of their high chemical durability and formability. However, photoluminescence dynamics of ns2-type ions in oxide glasses has not been well examined, even though they can exhibit high quantum efficiency. We report on the emission property of Sn2+-doped strontium borate glasses. Photoluminescence dynamics studies show that the peak energy of the emission spectrum changes with time because of site distribution of emission centre in glass. It is also found that the emission decay of the present glass consists of two processes: a faster S1-S0 transition and a slower T1-S0 relaxation and also that the energy difference between T1 and S1 states was found to be much smaller than that of (Sn, Sr)B6O10 crystals. We emphasize that the narrow energy gap between the S1 and T1 states provides the glass phosphor a high quantum efficiency, comparable to commercial crystalline phosphors.
Similar content being viewed by others
Introduction
Nowadays, various types of rare earth (RE)-doped phosphors are being actively developed worldwide for applications in light emitting diodes (LEDs)1, displays2, scintillators3, persistent luminescence4 and solid-state laser materials5. For example, phosphors for white LEDs have been enthusiastically examined6,7,8. These LEDs are expected to replace conventional white fluorescent lamps in the future because of their several advantages such as absence of Hg vapour, long life-span and low power consumption. Although deep-UV LEDs are not currently available commercially, recent developments in the field of LEDs clearly suggest a possibility of their realization in the near future9,10,11. However, the organic sealant for most types of powdered phosphors is damaged by high-power and/or high-energy excitation light sources. Consequently, it is expected that a monolithic material possessing high durability as well as good emission property will be required for future applications. Therefore, inorganic glasses exhibiting emission property are one of the practical phosphor candidates.
Investigation into the emission property of amorphous glasses has been mainly limited to RE-containing glasses12. This is not only because the analysis of RE cations whose emission is almost independent of the coordination state is relatively simple compared to other emission centres, but also because glass materials possessing random networks are thought to be unsuitable for a precise analysis of their luminescent property. In addition, oxide glasses have not attracted attention as phosphor candidates, despite possessing good formability and wide transparent wavelength region, because it is believed that such glasses exhibit low emission intensity. Therefore, the local structure of the emission centre in the amorphous glass has not been previously discussed, although its site distribution can vary.
Our group has focused on the ns2-type cations (n ≧ 4) such as Sn2+, Sb3+, Hg0, Tl+ and Pb2+ that give strong photoluminescence (PL) because of the parity allowed transition13,14. Because these types of emission centres possess electrons in the outermost shell in both the ground state (ns2) and the excited state (ns1np1), the emission spectrum is strongly affected by the coordination field. Therefore, it can be said that the amorphous glass, in which site distribution is much broader than that in a crystal, is one of the most fascinating solid-state host materials for an emission centre. Recently, we reported the emission property of the SnO-ZnO-P2O5 glass15,16,17,18,19,20. This glass showed blue light emission with high quantum efficiency (QE) caused by deep-UV irradiation. The emission is brought about by Sn2+, which is the most common ns2-type centre13. It is notable that the transparent glass containing no RE cations shows intense UV-excited emission that is comparable to a crystal phosphor such as MgWO4. Furthermore, the SnO-ZnO-P2O5 glass shows the highest efficiency among the glass materials that have no RE cations15. We have also demonstrated white light emission from the MnO-doped SnO-ZnO-P2O5 glass whose QE is comparable to MgWO416,17. Thus, the emission consisting of broad bands can be tailored by the addition of another emission centre such as Mn2+ or by changing the local coordination field (i.e., the chemical composition of the host glass) of the Sn2+ centre. Recently, we have also reported that the emission of the Sn2+ centre does not reflect the average (macroscopic) basicity of the glass; instead, it reflects the local basicity of the emission centre20. The result indicates that macroscopic randomness is not important for attaining high QE and that amorphous materials can exhibit high conversion efficiency comparable to that of crystalline phosphors by controlling the microscopic coordination state.
In the present study, we focus on a Sn-doped borate glass. Because the melting temperature required for fabricating borate glasses is generally lower than that required for conventional oxide glasses, partial oxidation of Sn2+ in the glass melt is expected, which results in a decrease in the emission efficiency. On the other hand, it is worthwhile to examine the PL of the Sn2+ emission centre in a random matrix instead of in phosphate glasses, because different host glasses provide the Sn2+ emission centre with different coordination fields. Neither the coordination state nor the emission mechanism of Sn2+ in a borate glass has yet been investigated; therefore, their investigation is attractive from scientific and industrial viewpoints. Because the Sn-doped SrB6O10 crystal is known to show emission by UV excitation21,22, it is expected that the Sn-doped borate glass also possesses a comparable emission property. For comparison, the chemical composition of the host glass was selected as 25.0SrO-75.0B2O3, which is equivalent to the stoichiometric composition of SrB6O10. Examining the effect of the randomness of the oxide matrix on the emission property of the Sn2+ emission centre is also meaningful from the viewpoint of the local structure in the glass matrix.
Results
Thermal property of SnO-doped strontium borate glasses
Transparent xSnO-(25.0-x)SrO-75.0B2O3 glasses were obtained in the region 0 ≤ x ≤ 1.8. The glass became translucent when x was increased to 1.9 because of the precipitation of SnO2. Tg of the nondoped 25SrO-75B2O3 glass was 610°C, which is approximately 200°C higher than previous phosphate glasses15 and decreased with increasing amount of SnO.
PL-PL excitation (PLE) spectra of SnO-doped strontium borate glasses
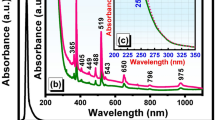
Figure 1 shows normalized PL-PLE spectra of xSnO-(25.0-x)SrO-75.0B2O3 glasses. Optical absorption spectra of these glasses are also shown. The PLE spectra were measured at the peak photon energy of each PL spectrum, whereas the PL spectra were measured by excitation at the peak energy of each PLE spectrum. The peak energy of the asymmetric broad emission slightly red-shifts with an increasing amount of SnO, although the peak width is almost unchanged. Because the SnO-free 25.0SrO-75.0B2O5 glass (x = 0) exhibiting the absorption edge at much higher photon energy (over 6 eV) shows no emission, it is clear that not only the emission band but also the observed absorption edge of the Sn-doped glass originates from the Sn2+ species. The main excitation band position locates in the vicinity of the optical absorption edge, which was also observed in the SnO-doped zinc phosphate glass system15,19. It is also found that the PLE spectra apparently consist of two bands; among them, the lower energy band strongly correlates with the optical absorption edge19. It was reported that the twofold-coordinated Sn in SiO2 shows two emission bands: α-band [singlet (S1) - singlet (S0), ~4.1 eV] and β-band [triplet (T1) - singlet (S0), ~3.1 eV]23. Although the T1 state consists of 3P0, 3P1 and 3P213, we use “T1” in the present paper, because the actual coordination number of Sn2+ to determine the transition probability has not yet been clarified. If we consider the origin of the emission based on the photon energy, the emission can be mainly attributed to the T1-S0 relaxation. The emission intensity has a maximum at composition x = 0.2, which is much smaller compared to that of a SnO-doped zinc phosphate glass system19. It is because overlapping of PL and PLE bands in the present borate system takes place even though SnO concentration was less than 1.0 mol% (see Fig. 1). The overlapping of PL and PLE bands induces concentration quenching to decrease emission intensity. On the other hand, the PLE band becomes broader with an increasing amount of SnO, while the peak energy is almost unchanged. Therefore, the absorption spectra and PLE bands of several Sn-containing glasses were examined to clarify in detail the correlation between the SnO amount and the PLE bands.
Figure 2(a) shows a PLE spectrum of the 1.5SnO-23.5SrO-75B2O3 glass together with the two bands deconvoluted using Gaussian functions: the higher energy excitation band A at 4.9 eV and the lower energy excitation band B at 4.2 eV. Figure 2 (b) shows the peak area ratio B/(A + B) and the peak energy of A and B bands as a function of SnO amount. With increasing SnO amount, the peak area ratio of the B band increases and the peak energy red-shifts. Note that the peak energy of the B band closely corresponds to the optical absorption edge estimated from the absorption spectra. It was reported that the twofold-coordinated Sn2+ species in SiO2 exhibits several excitation bands and the photon energy for the S0-S1 excitation is calculated as 4.9 eV23. Comparing this value with the present two values: 4.2 eV and 4.9 eV, it is expected that the excitation band A, whose peak position is independent of the SnO concentration, arises because of the S0-S1 excitation process of the twofold-coordinated Sn2+ species.
Concentration dependence of PLE spectra of xSnO-(25-x)SrO-75B2O3 glasses.
(a) PLE spectrum (emission at 3.13 eV) of 1.5SnO-23.5SrO-75B2O3 glass together with deconvoluted bands: higher energy band A and lower energy band B. (b) Peak position and area of B band as a function of SnO amount in PLE spectra of xSnO-(25-x)SrO-75B2O3 glasses.
Figure 3 shows the PL-PLE contour plots of the xSnO-(25.0-x)SrO-75.0B2O3 glasses: (a) x = 0.1, (b) 0.5 and (c) 1.0, using an intensity axis on a linear scale. The contour plot of the (Sn0.02Sr0.98) B6O10 crystal (d) is also shown for comparison. The emission intensity decreases with increasing amount of SnO, which is clearly because of the concentration quenching. These figures indicate that the higher the excitation energy, the higher the emission peak energy. In other words, the higher the S1 energy level, the higher the T1 level. Although a weak S0-T1 excitation band is observed at 3.9 eV in the (Sn0.02Sr0.98)B6O10 crystal21, indicated by the dashed circle in Fig. 3(d), no such excitation band is observed in the corresponding glasses. It is also obvious from Figs. 3(b) and (d) that both the excitation and emission bands are much broader in the glasses than in the corresponding crystal.
PL-PLE contour plots of xSnO-(25-x)SrO-75B2O3 glasses.
(a) x = 0.1, (b) x = 0.5 and (c) x = 1.0. Contour plot of (Sn0.02Sr0.98)B6O10 crystal (d) is also shown for comparison. Dashed circle in (d) shows weak excitation band owing to S0-T1 excitation21.
Photoluminescence dynamics of SnO-doped strontium borate glasses
The emission decay curves monitored at 2.95 eV for the xSnO-(25.0-x)SrO-75.0B2O3 glasses and the (Sn0.02Sr0.98)B6O10 crystal are shown in Fig. 4, where the samples were irradiated using UV light of 4.43 eV. The decay curves consist of two components: a faster component (lifetime, τ′1/e, of nanoseconds) and a slower component (τ″1/e of microseconds). From the decay curves of the glasses, τ″1/e was estimated as 7.4 ~ 5.6 μs, which decreases with increasing SnO amount as shown in Table 1. It seems that the emission decay of the microsecond order can be expressed using a single exponential curve and the emission has its origin in the Sn2+ emission centre. The decrease of decay constants suggests the concentration quenching occurs as shown in Fig. 1. Because the lifetime of the glasses is longer than that of the crystal (~4.0 μs)21, we have speculated that the structural disorder of the host material affects the lifetime of the Sn2+ emission centre. However, the faster decay was not observed in the SnO-ZnO-P2O5 glass system, which also showed the emission of Sn2+16,17,19. Therefore, it is expected that relaxation of the nanosecond order in the borate glass originates from the different coordination field of the Sn2+ centre in the phosphate glasses. As mentioned above, if we consider the origin of the emission based on the photon energy, we will find that the emission can be mainly attributed to the T1-S0 relaxation. However, when the presence of the faster relaxation is taken into account, whose decay time is shorter than 10 ns, it is expected that both the S1-S0 and T1-S0 relaxations exist. It turns out that the peak energy of the S1-S0 relaxation (~3.4 eV) in the present glass is much lower than that in the SiO2 glass (~4.1 eV).
Photoluminescence dynamics analysis using streak images
In order to confirm the existence of the faster emission decay, photoluminescence dynamics at room temperature was also evaluated using a streak camera and a monochromater. Figure 5a and 5b show the streak images of the 1.0SnO-24.0SrO-75.0B2O3 glass and (Sn0.02Sr0.98)B6O10 crystal, respectively. The vertical and horizontal axes show the timescale and photon energy, respectively. It is notable that timescale of the borate glass is nanosecond whereas that of crystal is microsecond. The image clearly shows the existence of fast decay in borate glass with a lifetime of less than 10 ns, which is different from conventional decay constant of Sn2+ centre in crystal with T1-S0 relaxation. The emission peaks of two materials are ~3.4 eV (glass) and 2.9 eV (crystal), suggesting these concerning energy levels are different. It also suggests that the emission peak energy of the glass slightly red-shifts, which is different from (Sn0.02Sr0.98)B6O10 crystal (see supplemental Fig. 1). Figure 5c shows the correlation between the emission peak energy and the time elapse of the 1.0SnO-24.0SrO-75.0B2O3 glass. The emission peak energy shifts toward lower energy with increasing time elapse, suggesting that the lower the emission peak energy, the longer the lifetime. Although the peak energy of the 1.0SnO-24.0SrO-75.0B2O3 glass is slightly higher than that of other glass systems, their time evolution is comparable. Because the internal QEs of the obtained glasses were at least 55% and that of the 0.2SnO-24.8SrO-75B2O3 glass was 81%, the main relaxation process is surely due to the photoluminescent decay. Because the Sn-free glass does not show such emission, we have attributed the observed emission decay of the nanoscale order to the S1-S0 relaxation of the Sn2+ centre, which has not been observed for phosphate glass systems. It is notable that internal QEs of the obtained glasses are higher than that of (Sn0.02Sr0.98)B6O10 crystal. This is also an advantage of the present Sn2+-doped glass phosphor.
Time-dependent emission property of SnO-SrO-B2O3 glasses and (Sn0.02Sr0.98)B6O10 crystal.
Streak images of 1.0SnO-24.0SrO-75B2O3 glass (a) and (Sn0.02Sr0.98)B6O10 crystal (b) excited by photon energy 4.86 eV. (c) Time-dependence of emission peak energy of xSnO-(25-x)SrO-75B2O3 glasses. Radiative relaxation of nanosecond order indicates relaxation from S1 excitation state.
Valence states of Tin in strontium borate glasses and crystal
Because the valence state of Sn is easily varied with the the preparation conditions, such as Sn concentration, preparation atmosphere and basicity of the host glass, it is important to examine the valence state of Sn in the matrix. Since Sr cation absorbs γ-ray of Sn, however, it is difficult to estimate Sn2+/Sn4+ ratio in these materials from the 119mSn Mössbauer spectroscopy. Therefore, X-ray absorption fine structure (XAFS) measurement is only one approach for the evaluation. Figure 6 shows Sn K-edge XANES spectra of several xSnO-(25.0-x)SrO-75.0B2O3 glasses (x = 0.5, 1.0 and 1.5) along with those of SnO and (Sn0.02Sr0.98)B6O10 crystal. Because a higher absorption edge indicates a higher oxidation state of the cation, we take absorption edge energy, E0, to be the energy at the zero-crossing of the 2nd derivative. The Sn K-edge energies of xSnO-(25.0-x)SrO-75.0B2O3 glasses are similar to that of SnO. Using each E0 value, |Δ(E0(SnO)-E0(glass))| is calculated to be less than 0.6 eV. Considering the resolution of the measurement (ΔE/E ~ 6 × 10−5), we assume that this difference (less than 1.75 eV) is insignificant. Thus, Sn K-edge XAFS supports the hypothesis that the percentage of Sn2+ to total Sn in xSnO-(25.0-x)SrO-75.0B2O3 glass is nearly 100%.
Discussion
RE-free emitting material is attractive not only from the viewpoint of the uneven distribution of RE on earth but also from the unique property24. The present results clearly suggest that Sn2+-containing glasses can be used as alternative inorganic phosphors that exhibit transparency in the visible region as well as a high QE. Although there are several reports on the luminescent property of Sn-containing glasses25,26,27,28,29, the reported quantum efficiencies were lower than that of our results. This is because these previous reports lack control of the Sn2+ cation that can give high QE because of the parity allowed transition. Because Sn2+ is a very sensitive and metastable species at higher temperatures, it is often reported that Sn2+ in a glass melt is easily oxidized during the melt in air condition. However, Sn K-edge XAFS measurement suggests that most of tin species in the present borate glass is Sn2+ that affects the emission property. We have confirmed that PLE spectrum of xSnO-(25.0-x)SrO-75.0B2O3 glasses melted in Ar atmosphere is similar to that of the one melted in air (see supplemental Fig. 2). It confirms that (1) percentage of Sn2+ to total Sn in xSnO-(25.0-x)SrO-75.0B2O3 glass is nearly 100%, (2) both excitation bands originate from Sn2+ centres. It was reported that there is an equilibrium reaction between Sn2+/Sn4+ and BO44−/BO33− as shown in Eq. (1),

Since11B MAS NMR spectrum of xSnO-(25.0-x)SrO-75.0B2O3 glass (see supplemental Fig. 3) consists of both BO44− and BO33− units, it is expected that Sn2+ species preferentially generated in the present melting condition (at 1100°C for 20 min) even though melting was performed in air. The effect of the equilibrium reaction on the valence state of tin will be clarified in a separated paper by comparison with the glass melted in Ar and that of the one melted in air.
As shown in Figs. 1–2, the excited state of Sn2+ in borate glasses consists of two bands. Although two excitation bands attributed to the Sn2+ emission centre in glass materials have been reported19,20,21, there are conflicting assignments of the excitation bands. Considering the previous finding that the Sn2+ centre in a silica glass with the C2v symmetry shows the S1 band at 4.9 eV21 and the present finding of the high internal QEs (at least 55%), we assume that there are two different Sn2+ emission centres that take different coordinate states17. According to a previous report concerning Sn2+ in zinc phosphate glasses17, we call the higher energy band (A) the S1 band and the lower band (B) the S1′ band. The S1 band at 4.8–4.9 eV, whose energy is comparable to the previous value21, is almost unchanged, whereas the lower band S1′ red-shifts with an increasing amount of SnO, as shown in Fig. 2. The emergence of the S1′ band is also observed in the PL-PLE contour mappings (Fig. 3), in which the lower excitation band is observed in the glass containing a high amount of SnO. Therefore, it is suggested that the S1′ band originates in a local structure different from the one that yields the S1 band (two coordinated Sn2+ centre with C2v symmetry) that occurs at low Sn2+ concentration. However, the peak area of the S1′ band in the borate glass is much smaller than that in the phosphate glass containing the same SnO concentration17. Because the network randomness of phosphate glasses is higher than that of borate glasses, it is expected that not only a high ionic network but also the network flexibility are responsible for high SnO concentration and the existence of the S1′ state. Although the actual coordination of Sn2+ at the S1′ state in the borate glass is unknown, we believe that the S1′ state is characteristic of the glass phosphor exhibiting high QE.
In the present study, we assume that emission at the microsecond scale originates during the relaxation from the T1 state, although the actual spin state has not been measured. Similarly, based on the timescale, it is assumed that the emission occurs from the S1 state. We have recently reported that the emission of Sn2+ is affected by the local coordination field18 and not by the average basicity of glasses. It is expected that a nonuniform distribution of the Sn2+ emission centre in the glass causes a wide distribution of the excitation energy and lifetime. An expected large distribution of the S1 state of the Sn2+ emission centre in the glass is actually observed in the PL-PLE contour mapping and the time-dependent emission decay curves. In particular, the streak images, with a lifetime of nanoseconds, show that the time-integrated PL spectra consist of asymmetric emission exhibiting nonexponential decay at the nanosecond order. Because radiative relaxation at the nanosecond order has not been reported in Sn2+-doped crystals or in Sn2+-doped phosphate glasses, we emphasize that the observed relaxation depends on the borate glass system. In addition, it is notable that emission peak energy of S1–S0 relaxation is close to that of T1–S0 relaxation. Although it is expected that the difference originates from both the symmetry and basicity of local coordination field of Sn2+ centre, it has not been clarified yet. Further study is needed for understanding the basic science to improve the performance.
Based on these results, we discuss the emission mechanism of the xSnO-(25.0-x)SrO-75.0B2O3 glass. Figure 7 shows plausible energy schemes for the photoluminescence process of Sn2+ in the (Sn0.02Sr0.98)B6O10 crystal19 (a), Sn-doped SiO2 glass21 (b) and 0.5SnO-24.5SrO-75.0B2O3 glass (c). In the case of the (Sn0.02Sr0.98)B6O10 crystal, photon energies for the S0–S1 and S0–T1 excitations are 4.8 and 3.9 eV, respectively. The observed emission at 2.9 eV is attributed to the T1–S0 relaxation. However, the photon energy for the S0–S1 excitation in the two glasses is 4.9 eV, which is slightly higher than that in the corresponding crystal. In the glass material, there are two decay processes: one corresponding to a faster relaxation from the S1 state and the other corresponding to a slower one from the T1 state. Although the emission energy from the T1 state of silica glass is similar to that of the present borate glass, the emission energy from the S1 state of the former is higher than that of the latter. It is easily understood that the smaller the energy difference between the S1 and T1 states, the more effective the intersystem crossing will be to prevent nonradiative transition. Therefore, it is expected that the high QE of the present glass, which is comparable to the crystalline phosphor, is attained by the unique energy scheme shown in Figure 7c. Most previous works on photoluminescence of ns2-type emission centre were performed in alkali halide systems30,31,32. Although it has been thought that ns2-type ion takes Oh symmetry in both halides and oxide, it is natural that the emission centre possessing the lone pair electrons exhibits more disordered symmetry in glass (random) materials. We have, therefore, emphasized that ns2-type centre in random materials possessing unique emission property will open up an application field for emitting materials.
Plausible energy scheme for photoluminescence process of Sn2+ emission centre.
(a) (Sn0.02Sr0.98)B6O10 crystal21, (b) Sn2+-doped silica glass23 and (c) 0.5SnO-24.5SrO-75B2O3 glass. Solid lines and dashed lines indicate radiative and nonradiative transitions, respectively. Photon energies of each excitation (blue) and relaxation (red) processes are shown.
In summary, we have demonstrated the UV-excited light emission property of Sn2+-doped strontium borate glasses. Two excitation bands emerged depending on the amount of SnO, where the lower energy band strongly correlates with the optical absorption edge. The broad emission of the glass is caused by the S1-S0 and T1-S0 relaxation processes of Sn2+ centre. The randomness of the amorphous matrix makes the emission decay duration longer compared with the corresponding crystal. It is notable that the energy difference between the S1 and T1 states is much narrower in the glass than the corresponding crystal, which may give the glass phosphor a high QE, comparable to the commercial crystalline phosphor. The present paper demonstrates a possibility of unique emission property of ns2-type centre in a random matrix, which will be different from the conventional emission system in halide crystals.
Methods
Preparation of SnO-doped strontium borate glass
The xSnO-(25.0-x)SrO-75.0B2O3 glasses were prepared according to a conventional melt-quenching method using a platinum crucible. Batches consisting of SnO (99.5%), SrCO3 (99.9%) and B2O3 (99.9%) were mixed and melted at 1100°C for 20 min. The glass melt was quenched on a stainless steel plate maintained at 200°C and then annealed at the glass transition temperature, Tg, as measured by the differential thermal analysis (DTA), for 1 h. After cutting (10 mm × 10 mm × 1 mm), the glass samples were optically polished with an aqueous diamond slurry.
Analysis methods. The absorption spectra were measured using a U3500 UV-vis-NIR spectrophotometer (Hitachi, Japan). The photoluminescence (PL) and PL excitation (PLE) spectra were measured at room temperature using an 850 fluorescence spectrophotometer (Hitachi, Japan). Band pass filters were used for the excitation (5 nm) and the emission (3 nm) for PL measurements. The emission decay at room temperature was measured using a Quantaurus-Tau (Hamamatsu Photonics, Japan). The excitation light source used was an LED operated at photon energy 4.43 eV and frequency 10 kHz. The PL dynamics was also evaluated using a streak camera and a monochromater. The light source used for photoexcitation was an optical parametric amplifier system based on a regenerative amplified mode-locked Ti:sapphire laser (Spectra Physics, USA) with a pulse duration of 150 fs and a repetition rate of 1 kHz. The photon energy was 4.96 eV, which corresponded to the peak energy of the PLE spectra. The Sn K-edge (29.3 keV) XAFS spectra were measured at BL01B1 of SPring-8 (Hyogo, Japan). The storage ring energy was operated at 8 GeV with a typical current of 100 mA. The measurements were carried out using a Si (311) double crystal monochromator in the transmission mode (Quick Scan method) at r.t.
Preparation of SnO-doped strontium borate crystal. For comparison, a (Sn0.02Sr0.98)B6O10 crystal was prepared by sintering. According to a previous report21, the starting materials of SnO, SrCO3 and B2O3 were mixed and heat-treated twice at 800°C in a N2 atmosphere. A pellet of the sintered (Sn0.02Sr0.98)B6O10 crystal (10 mm in diameter) was used for the measurements.
References
Nakamura, S., Mukai, T. & Senoh, M. Candela-class high-brightness InGaN-AlGaN double-heterostructure blue-light-emitting diodes. Appl. Phys. Lett. 64, 1687–1689 (1994).
Downing, E., Hesselink, L., Ralston, J. & Macfarlane, R. A three-color, solid-state, three-dimensional display. Science 273, 1185–1189 (1996).
Nikl, M. Wide band gap scintillation materials: Progress in the technology and material understanding. Phys. Stat. Solidi A 178, 595–620 (2000).
Matsuzawa, T., Aoki, Y., Takeuchi, N. & Murayama, Y. New long phosphorescent phosphor with high brightness, SrAl2O4:Eu2+, Dy3+. J. Electrochem. Soc. 143, 2670–2673 (1996).
Geusic, J. E., Marcos, H. M. & Vanuitert, L. G. Laser oscillations in Nd-doped yttrium aluminum yttrium gallium and gadolinium garnets. Appl. Phys. Lett. 4, 182–184 (1964).
Blasse, G. & Bril, A. Investigation of some Ce3+-activated phosphors. J. Chem. Phys. 47, 5139–5145 (1967).
Kim, J. S. et al. Warm-white-light emitting diode utilizing a single-phase full-color Ba3MgSi2O8:Eu2+, Mn2+ phosphor. Appl. Phys. Lett. 84, 2931–2933 (2004).
Daicho, H. et al. A novel phosphor for glareless white light-emitting diodes. Nature Commun. 3, 10.1038/ncomms2138 (2012).
Oto, T., Banal, R. G., Kataoka, K., Funato, M. & Kawakami, Y. 100 mW deep-ultraviolet emission from aluminium-nitride-based quantum wells pumped by an electron beam. Nature Photon. 4, 767–771 (2010).
Khan, A., Balakrishnan, K. & Katona, T. Ultraviolet light-emitting diodes based on group three nitrides. Nature Photon. 2, 77–84 (2008).
Taniyasu, Y., Kasu, M. & Makimoto, T. An aluminium nitride light-emitting diode with a wavelength of 210 nanometres. Nature 441, 325–328 (2006).
Tanabe, S. Optical transitions of rare earth ions for amplifiers: how the local structure works in glass. J. Non-Cryst. Solids 259, 1–9 (1999).
Yen, W. M., Shionoya, S. & Yamamoto, H. Phosphor handbook 2nd edition (CRC Press, Boca Raton, 2007).
Fukuda, A. Jahn-Teller fffect on the structure of the emission produced by excitation in the A band of KI:Tl-type phosphors. two kinds of minima on the Γ4− (3T1u) adiabatic potential-energy surface. Phys. Rev. B 1, 4161–4178 (1970).
Masai, H., Takahashi, Y., Fujiwara, T., Matsumoto, S. & Yoko, T. High photoluminescent property of low-melting Sn-doped phosphate glass. Appl. Phys. Express 3, 082102 (2010).
Masai, H. et al. White light emission of Mn-doped SnO-ZnO-P2O5 glass containing no rare earth cation. Opt. Lett. 36, 2868–2870 (2011).
Masai, H. et al. High efficient white light emission of rare earth-free MnO–SnO–ZnO–P2O5 glass. J. Ceram. Soc. Jpn. 119, 2868–2870 (2011).
Masai, H. et al. Fabrication of Sn-doped zinc phosphate glass using a platinum crucible. J. Non-Crystal. Solids 358, 265–269 (2012).
Masai, H. et al. Correlation between emission property and concentration of Sn2+ centre in the SnO-ZnO-P2O5 glass. Opt. Express 20, 27319–27326 (2012).
Masai, H. et al. Localized Sn2+ emission centre independent of the optical basicity of zinc phosphate glass. Chem.Lett. 42, 132–134 (2013).
Leskelä, M., Koskentalo, T. & Blasse, G. Luminescence Properties of Eu2+, Sn2+ and Pb2+ in SrB6010 and Sr1−xMnxB6O10 . J. Solid State Chem. 59, 272–279 (1985).
Koskentalo, T., Leskelä, M. & Niinistö, L. Studies on the luminescence properties of manganese activated strontium borate SrB6O10 . Mater. Res. Bull. 20, 265–274 (1985).
Skuja, L. Isoelectronic series of twofold coordinated Si, Ge and Sn atoms in glassy SiO2: a luminescence study. J. Non-Cryst. Solids 149, 77–95 (1992).
Pan, Z., Lu, Y.-Y. & Liu, Y. Sunlight-activated long-persistent luminescence in the near-infrared from Cr3+-doped zinc gallogermanates. Nature Mater. 11, 58–63 (2012).
Parke, S. & Webb, R. S. Optical properties of Sn2+ and Sb3+ in calcium metaphosphate glass. J. Phys. D. 4, 825–828 (1971).
Reisfeld, R., Boehm, L. & Barnett, B. Luminescence and nonradiative relaxation of Pb2+, Sn2+, Sb3+ and Bi3+ in oxide glasses. J. Solid State Chem. 15, 140–150 (1975).
Sobha, K. C. & Rao, K. J. Investigations on sodium tin phosphate and tin pyrophosphate glasses. Proc Indian Acad Sci Chem Sci. 107, 573–580 (1995).
Ehrt, D., Leister, M. & Matthai, A. Polyvalent elements iron, tin and titanium in silicate, phosphate and fluoride glasses and melts. Phys. Chem. Glasses 42, 231–239 (2001).
Qiu, J. & Makishima, A. Ultraviolet-radiation-induced structure and long-lasting phosphorescence in Sn2+–Cu2+ co-doped silicate glass. Sci. Technol. Adv. Mater. 4, 35–38 (2003).
Toyozawa, Y. & Inoue, M. Dynamical Jahn-Teller effect in alkali halide phosphors containing heavy metal ions. J. Phys. Soc. Jpn. 21, 1663–1679 (1966).
Fukuda, A. & Yuster, P. Unusually large change in radiative lifetime of A-band emission in KI -In+ and KI-Sn2+ induced by a magnetic-field. Phys. Rev. Lett. 28, 1032–1034 (1972).
Kamishina, Y. et al. Emission-spectroscopy of Alkali-halide phosphors doped with s2 ions: KI-Sn2+. Phys. Rev. B 22, 3010–3020 (1980).
Acknowledgements
This work was partially supported by the Asahi Glass Foundation, the Inamori Foundation, the Kazuchika Okura Memorial Foundation and Research Institute for Production Development. The work was also supported by ICR Grants for Young Scientists and the Sumitomo Electric Industries Group CSR Foundation (to Y.Y. and Y. K.). The author (H.M.) thanks Prof. A. Wakamiya (Institute for Chemical Research, Kyoto University) and Prof. T. Yanagida (Kyushu Institute of Technology) for allowing measurement of emission decay and quantum efficiency.
Author information
Authors and Affiliations
Contributions
H.M. and T.Y. designed the research. H.M. and Y.S. executed the materials preparation and measurement except luminescent dynamics and XAFS. H.M. and K.T. examined XAFS analysis. Y.Y. and Y.K. measured the luminescent dynamics. H.M. and T.Y. wrote the paper. All authors discussed the results.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplemental Figures
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Masai, H., Yamada, Y., Suzuki, Y. et al. Narrow Energy Gap between Triplet and Singlet Excited States of Sn2+ in Borate Glass. Sci Rep 3, 3541 (2013). https://doi.org/10.1038/srep03541
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep03541
This article is cited by
-
Thermally stable multi-color emitting Dy3+/Eu3+ co-doped BaO–ZnO–Li2O–P2O5 glasses for w-LEDs
Journal of Materials Science: Materials in Electronics (2023)
-
Down-shifting photoluminescence studies of thermally stable Dy3+ ions doped borosilicate glasses for optoelectronic device applications
Journal of Materials Science: Materials in Electronics (2022)
-
Dy3+-doped P2O5–Al2O3–K2O–CaF2–LiF glasses: thermal, spectroluminescence and photometric properties
Bulletin of Materials Science (2022)
-
Validity of Valence Estimation of Dopants in Glasses using XANES Analysis
Scientific Reports (2018)
-
Photoluminescence of monovalent indium centres in phosphate glass
Scientific Reports (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.