Abstract
Antarctic krill (Euphausia superba) is a key component of the Southern Ocean food web. It supports a large number of upper trophic-level predators and is also a major fishery resource. Understanding changes in krill abundance has long been a priority for research and conservation in the Southern Ocean. In this study, we performed stable isotope analyses on ancient Adélie penguin tissues and inferred relative krill abundance during the Holocene epoch from paleodiets of Adélie penguin (Pygoscelis adeliae), using inverse of δ15N (ratio of 15N/14N) value as a proxy. We find that variations in krill abundance during the Holocene are in accord with episodes of regional climate changes, showing greater krill abundance in cold periods. Moreover, the low δ15N values found in modern Adélie penguins indicate relatively high krill availability, which supports the hypothesis of krill surplus in modern ages due to recent hunt for krill-eating seals and whales by humans.
Similar content being viewed by others
Introduction
The Southern Ocean is biologically the world's most productive ocean. At the hub of the Antarctic marine food web, krill is the primary consumer of diatoms, the major prey for many species of fishes, penguins, seals and whales1,2,3 and a substantial commercial fishery resource1. The abundance of krill is very sensitive to climate change and has significant impacts on high trophic-level predators in the Southern Ocean ecosystems4,5. Recent Antarctic krill populations have been significantly influenced by rapid climate change, human removal of krill-eating predators and intense commercial fishing1,6,7. Therefore, the records of krill population change, especially those pre-dating the onset of human harvesting in Antarctic, are crucial for understanding and predicting responses of krill population to natural climate changes.
Modern krill abundance can be obtained directly from acoustic and net surveys. Krill population data from scientific trawls are available for the past ~30 years6. For historical krill abundance, only about 100 years of data have been inferred from Antarctic fur seals8. The long-term historical krill abundance prior to human intervenes remains unknown. Similar to Antarctic fur seal, Adélie penguin is an important land-based krill predator, which has been chosen as an indicator of changes in krill availability and abundance by the commission for the conservation of Antarctic marine living resources9. Furthermore, Adélie penguin is a circum-Antarctic distributed species; continuous and long time-series remains of Adélie penguin could be preserved in various lake sediments10. Thus, Adélie penguin is an ideal indicator for tracking krill availability and abundance over a long period of time.
Stable isotope analysis of animal tissues is a powerful tool for examining wildlife foraging habitats, diet and migration patterns11,12,13, especially in historical periods14,15. Keratinous tissues such as toe nails, feathers and hair and bone collagen can preserve dietary information for long periods of time16, particularly in the cold and dry Antarctic environment. They are ideal for investigating paleodiets of krill predators8. Hairs and faeces in lake sediments have been used successfully to infer past population dynamics of seals and penguins in Antarctica17,18,19. Similar stable isotopes and biomarkers have also been used extensively in northern high-latitude or Arctic regions to study the effects of climate change, seabird colonisation and past whaling activities on lake ecosystems20,21,22.
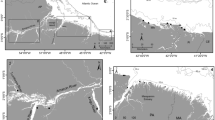
Here, we analyzed stable nitrogen isotope ratios (15N/14N, expressed as δ15N) of modern and ancient Adélie penguin bones and feathers from the Vestfold Hills, East Antarctica (Figure 123,24 and Figure 225), with the main aim of inferring the relative krill abundance over a long historical time. Several observations follow. First, δ15N values in penguin tissues show an enrichment of ~3–5‰ from prey to predator in marine ecosystems11. Second, as a diet of penguins, krill are much lower in δ15N values than fishes are15. Third, Adélie penguins feed preferentially on krill species26,27,28 of Euphausia superba in the Antarctic Peninsula and of Euphausia crystallorophias along the East Antarctic coasts. Lower δ15N values in Adélie penguins indicate diets based primarily on krill, while higher values indicate diets richer in fishes and other species of potentially higher trophic level (e.g., squid). The variations in δ15N values of penguin tissues indicate the changes in the proportion of krill in penguin diets and thus the krill availability and abundance in the foraging area29. The δ15N values in penguin tissues through time could serve as a proxy for krill availability and abundance in foraging areas.
Adélie penguin in the Vestfold Hills and the bones and feathers from the sediment core DG4 profile with conventional AMS 14C dates25 (yr BP: years before present).
Photo credit: T. Huang.
Results
The δ15N values of penguin bones and feathers at different depths of a sediment core DG4, as well as modern samples from Magnetic, Zolotov and Gardner Island at Vestfold Hills, are given in Table 1. The δ15N values of main preys for Adélie penguin in East Antarctic30,31 are plotted in Figure 3. Sample sizes (n) reported below are the numbers of the distinct depths in the DG4 sediment at which samples were collected, as shown in Table 1. Modern Adélie penguins at Vestfold Hills have δ15N value (mean ± standard error of mean) of 10.1 ± 0.3‰ for bones (n = 6) and 10.0 ± 0.04‰ for feathers (n = 6), which are very close to those reported in Adélie Land (9.4 ± 0.09‰, n = 20) and MacRobertson Land (9.4 ± 0.2‰, n = 31), East Antarctica23,24 (Figure 3). The δ15N values of ancient Adélie penguins are much higher. They ranged from 12.5‰ to 18.1‰ in the bones with a mean of 15.3 ± 0.6‰ (n = 10) and ranged from 11.3‰ to 15.5‰ with a mean of 13.6 ± 0.4‰ (n = 11) in the feathers (Table 1 and Figure 4).
δ15N values (mean ± standard error of mean) of Adélie penguins in this study and previous studies23,24 and penguin prey δ15N values compiled by Cherel et al. (2008)30 for Euphausia crystallorophias, Euphausia superba and Pleuragramma antarcticum and Gillies et al. (2012)31 for Trematomus bemacchii.
(VH: Vestfold Hills, AL: Adélie Land, ML: MacRobertson Land).
δ15N value fluctuations in Adélie penguin bones and feathers during the Holocene epoch, along with the episodes of climate change as inferred from ice cores and marine sediments in East Antarctic32,33,34.
The instrument error for the isotope analysis is ≤0.2‰. Red bands refer to warm climate periods and blue bands to cold periods and expanded sea ice condition.
The δ15N values in modern Adélie penguin bones and feathers at Vestfold Hills are similar (Wilcoxon rank sum test statistic T = 45, p = 0.35). For the same type of tissues, δ15N in modern and ancient samples are significantly different (for bones: T = 115, p = 0.0002 and for feathers: T = 132, p = 0.0002), with higher δ15N found in ancient penguins.
In addition, the δ15N values in ancient penguin feathers and bones between warm and cold climate conditions are also significantly different. Here, we determined the episodic warm periods (7500–6300 yr BP and 4800–2200 yr BP) and cold periods (8500–7600 yr BP and 6300–5700 yr BP) according to previously reconstructed climate change during the Holocene in East Antarctic based on ice cores32 and marine sediment cores33,34. Using information of warm/cold periods provided in Table 1, we were able to compare δ15N values in cold periods against those in warm periods. It was found that δ15N values of ancient Adélie penguin during cold periods are much lower than those during warm periods (Figure 4), in both bones (T = 3, p = 0.05, nwarm = 7, ncold = 2) and feathers (T = 10, p = 0.01, nwarm = 6, ncold = 4). Furthermore, when combining data from bones and feathers to form a single test, we observed even stronger evidence (T = 21, p = 0.0001, from a tissue-stratified Wilcoxon rank sum test). This implies the relative krill abundance in the cold periods is much higher as compared with that in the warm periods.
Discussion
Different penguin tissues have different metabolic rates and their stable isotope signatures have different time scales, from days for blood plasma to weeks for feathers and to months of average turnover-time for collagen of bones16. Because our samples were collected at a breeding site during a summer season, the stable isotope values of archive feathers in the sediment core DG4 reflect those of the average diets at the time of feather growth. The nitrogen isotope values of penguin bones reflect those of the average diets of Adélie penguins during the last turnover of their bone collagen. Visual inspection showed that the ancient feathers in DG4 were from adult penguins whereas the source was uncertain for the bones (which can be from adults and/or juveniles). Since the trend and magnitude of δ15N in the ancient penguin feathers are similar to those in the bones (Figure 4), the possible age difference in the penguin bones seems unlikely to drive the temporal change of δ15N during the Holocene epoch.
Variation of the inferred relative krill abundance (represented by the inverse of δ15N values in penguin tissues) was found to be in accordance with previously reconstructed episodes of climate change during the Holocene in East Antarctic32,33,34. That is, periods with high krill abundance (lower δ15N values) correspond to cold climates32,33, whereas periods with low krill abundance (higher δ15N values) correspond to warm climates32,34. Krill is a stenothermal, cold-water planktonic species and is very sensitive to natural climate changes. Krill abundance has been linked with expanded sea ice cover (extent and duration) and increased latitudinal extent of cool water6,35,36, which are consistent with our findings. Moreover, our results from Adélie penguins are also consistent with those from Antarctic fur seal hairs from a sediment core spanning the past century in the West Antarctic Peninsula8. Taken together, these results suggest that the link between krill abundance and climate persists over the past 8,000 years in East Antarctica.
It has been reported37 that δ15N values of the primary producer (baseline) and thus penguins' preys may also be influenced by climates. This can in turn give rise to the change of δ15N in penguins in response to varied climates. However, we note that the δ15N values in the Southern Ocean phytoplankton (fragilariopsis kerguelensis, main food of Antarctic krill) are higher during cold climate periods and lower during warm periods38, opposite to the patterns seen in our penguin tissues. Based on these facts, we argue that the temporal variations of δ15N in penguin tissues cannot be attributed to the variation of δ15N of the krill themselves but primarily to the change of penguin's diet composition.
Modern penguin bones and feathers have much lower δ15N values than ancient ones. Similar low δ15N values were also observed in modern penguin eggshells by Emslie & Patterson (2007)15. These differences are most likely caused by substantial dietary changes between modern and ancient Adélie penguins (Figures 3, 4). There are two other possibilities which, however, do not seem to explain the differences observed in the current study. First, diagenetic processes may change dietary signals in tissues over time. This is unlikely for penguin feathers and bones because of the excellent preservation condition in the dry and cold Antarctic environment39 though the preservation state of ancient tissues has not been directly studied in the present study. Second, ammonia volatilization could cause older tissues to become more enriched in nitrogen-15 than younger or modern tissues40. This, however, only applies to sediment, soil and plants growing in the soil. In fact, the ancient penguin bones and feathers in DG4 have much lower δ15N values (12–18‰) than those in the sediments of DG4 core (25–39‰, Table S1) and ornithogenic soils (~30‰) (Mizutani & Wada, 1988)40. Furthermore, this would not explain the similar patterns of fluctuations in δ15N values observed in both feathers and bones if volatilization is a significant factor (see also Emslie et al. 201341).
Taken together, the lower δ15N values of modern tissues than in ancient tissues indicate higher proportion of krill in modern Adélie penguin diets and thus greater krill abundance in modern ages than in ancient ages, in the East Antarctic. This is consistent with the observations by Emslie & Patterson (2007)15, which supports the ‘krill surplus’ hypothesis, i.e., the higher krill abundance is likely due to the hunting of krill-eating seals and whales since as early as the 19th century.
One limitation of the present study is the small sample size for analysis. In the sediment core DG4, each 1-cm section contains only one or two pieces of feathers and bones. To address this issue, we have chosen a nonparametric statistical method to test the differences in δ15N values between different conditions. Since Adélie penguins and krill are widely distributed in the circum Antarctic, we expect to find more sampling sites and collect multiple sediments in future field work.
Our results corroborate that climate change has a pronounced effect on krill abundance and hence Adélie penguin diet composition26,27,28. The reported 8000-year record of the relative krill abundance, as inferred from Adélie penguin paleodiets, provides a unique insight into the change of regional marine food chains in natural status. This will be valuable for assessing impacts of future climate changes on this key species and thus for the conservation and management of Antarctic marine resources.
Methods
Sample collection and chronology
We collected a sediment core DG4 near a large Adélie penguin colony at Gardner Island, Vestfold Hills (Figure 1), East Antarctica, during the 2005–2006 austral summer. There are a large number of penguins in the colony in summer breeding seasons and penguin remains such as guanos, bones and feathers are deposited into the sediments each year (Figure 2). This sediment thus preserves a record of penguin activities at this colony since it was first formed. The core was sectioned at 1 cm intervals in the lab. Layers from 55 cm to the surface contained numerous penguin bones and feathers, but sample sizes per 1 cm section were quite small. Modern bone and feather samples were collected from dead adult penguins at Gardner, Magnetic and Zolotov Island, Vestfold Hills.
The chronology of DG4 has been established by AMS 14C dates on penguin tissues and bulk sediments in the core in a previous study25 and the penguin occupation appears to have been continuous since 8500 year BP to the present25.
Stable isotope analyses
Penguin bones and feathers were picked from each 1-cm section of the sediment core and used for stable isotope analysis. These samples as well as the modern adult penguin tissues were cleaned with Millipore water and 2:1 chloroform: methanol solution and then dried in an oven at 40°C. The cleaned samples were cut into small pieces and weighed in a tin capsule. Stable nitrogen isotope ratios in whole feathers and bone collagens were determined using isotope ratio mass spectrometer at the G.G. Hatch Isotope Laboratories, Earth Sciences, University of Ottawa, with a precision at ≤0.2‰. Isotope ratio (15N/14N) in samples are reported in δ notation and the units are per mil (‰) and defined as δ15N = [(Rsample − Rstandard)/Rstandard], where Rsample is isotope ratio of the sample and Rstandard of the atmosphere (See Supplementary Information for more detail).
δ15N as a proxy for krill abundance
We used the inverse of isotopic ratio of nitrogen, δ15N, in penguin tissues as a qualitative proxy for krill abundance. The δ15N in tissues has been used to reconstruct diet composition of marine species13,14,15. Generally, marine predators are 15N-enriched over their diet, because of the kinetic isotope effects in metabolic pathways involving the bond-breaking or synthesis of biochemical compounds, during which the molecules that contain the lighter nitrogen-14 are preferentially utilized over the same molecules containing the heavier nitrogen-1511. δ15N values can therefore be used as an indicator for the dietary change of marine predators.
Information of krill population can be estimated from penguin's diets based on traditional methods such as stomach lavage analyses42 which, however, often come with biases associated with sampling of gut contents. The stable nitrogen isotope method as used here can be regarded as a qualitative biochemical method and reflects the average amount of all the diets rather than specific ones. Since this method assesses paleodiets of wildlife through their ancient tissues8, there is no time limitation regarding availability of diets.
Adélie penguins feed primarily on krill and, when krill are scarce, on fishes26,27,28. Because krill are much more 15N-depleted than fishes15, δ15N in penguin tissues where diet information is well preserved can indicate the changes of the proportion of krill and thus their abundance. Specifically, high δ15N indicates low krill abundance and low δ15N indicates high krill abundance.
Statistical test for differences in δ15N between different conditions
To assess the significance of the difference in penguin δ15N isotope values between different time periods (modern vs. ancient), different tissues (feather vs. bone) and different reconstructed climate conditions (warm vs. cold), we adopted the nonparametric Wilcoxon rank sum test. Due to small samples sizes and presence of ties, asymptotic p value resulting from a Normal distribution approximation is invalid. Therefore, we used an exact two-sided p value obtained via a permutation procedure (i.e., randomly reassigning δ15N values across groups of samples) for each test as reported in “Results”. The function “wilcox_test” in the R package “coin” was used to perform these tests. The function also implements an extension of the Wilcoxon rank sum test to allow for stratifying on another factor. We've utilized this technique to compare δ15N in cold periods vs. δ15N in warm periods while accounting for the possible impact of tissues (bone or feather) on δ15N.
References
Everson, E. Krill. Biology, Ecology and Fisheries (Blackwell Science, London, 2000).
Hofmann, E. E. & Murphy, E. J. Advection, krill and Antarctic marine ecosystems. Antarct Sci 16, 487–499 (2004).
Nicol, S. Krill, Currents and Sea Ice: Euphausia superba and Its Changing Environment. Bioscience 56, 111–120 (2006).
Croxall, J. P. Southern-ocean environmental changes: effects on seabird, seal and whale populations. Phil. Trans. R. Soc. B 338, 319–328 (1992).
Trivelpiece, W. Z. et al. Variability in krill biomass links harvesting and climate warming to penguin population changes in Antarctica. Proc. Natl. Acad. Sci. USA 108, 7625–7628 (2011).
Atkinson, A., Siegel, V., Pakhomov, E. & Rothery, P. Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature 432, 100–103 (2004).
Nicol, S., Foster, J. & Kawaguchi, S. The fishery for Antarctic krill-recent developments. Fish. Fish 13, 30–40 (2012).
Huang, T. et al. Relative Changes in Krill Abundance Inferred from Antarctic Fur Seal. PLoS ONE 6(11), e27331 (2011).
Agnew, D. J. The CCAMLR Ecosystem Monitoring Programme. Antarct. Sci. 9, 235–242 (1997).
Sun, L. G. et al. Vertebrate records in polar sediments: biological responses to past climate change and human activities. Earth-Sci Rev 126, 147–155 (2013).
Hobson, K. A., Piatt, J. F. & Pitocchelli, J. Using Stable Isotopes to Determine Seabird Trophic Relationships. J. Anim. Ecol. 63, 786–798 (1994).
Cerling, T. E. et al. 2006 Stable isotopes in elephant hair document migration patterns and diet changes. Proc. Natl. Acad. Sci. USA 103, 371–373 (2006).
Cherel, Y., Kernaleguen, L., Richard, P. & Guinet, C. Whisker isotopic signature depicts migration patterns and multi-year intra- and inter-individual foraging strategies in fur seals. Biol. Lett. 5, 830–832 (2009).
Hilton, G. M. et al. A stable isotopic investigation into the causes of decline in a sub-Antarctic predator, the rockhopper penguin Eudyptes chrysocome. Global Change Biol 12, 611–625 (2006).
Emslie, S. D. & Patterson, W. P. Abrupt recent shift in delta C-13 and delta N-15 values in Adélie penguin eggshell in Antarctica. Proc. Natl. Acad. Sci. USA 104, 11666–11669 (2007).
Bearhop, S., Furness, R. W., Hilton, G. H. & Waldron, S. A forensic approach to understanding diet and habitat use from stable isotope analysis of (avian) claw material. Funct. Ecol. 17, 270–275 (2003).
Hodgson, D. A. & Johnston, N. M. Inferring seal populations from lake sediments. Nature 387, 30–31 (1997).
Sun, L. G., Xie, Z. Q. & Zhao, J. L. Palaeoecology-A 3,000-year record of penguin populations. Nature 407, 858–858 (2000).
Sun, L. G. et al. A 1,500-year record of Antarctic seal populations in response to climate change. Polar Biol. 27, 495–501 (2004).
Smol, J. P. et al. Climate-driven regime shifts in the biological communities of Arctic lakes. Proc. Natl Acad. Sci. USA 102, 4397–4402 (2005).
Michelutti, N. et al. Seabird-driven shifts in Arctic pond ecosystems. Proc. R. Soc. B 276, 591–596 (2009).
Douglas, M. S. V., Smol, J. P., Savelle, J. M. & Blais, J. M. Prehistoric Inuit whalers affected Arctic freshwater ecosystems. Proc. Natl. Acad. Sci. USA 101, 1613–1617 (2004).
Jaeger, A. & Cherel, Y. Isotopic Investigation of Contemporary and Historic Changes in Penguin Trophic Niches and Carrying Capacity of the Southern Indian Ocean. PLoS ONE 6, e16484 (2011).
Tierney, M., Southwell, C., Emmerson, L. M. & Hindell, M. A. Evaluating and using stable-isotope analysis to infer diet composition and foraging ecology of Adélie penguins Pygoscelis adeliae. Mar Eco Prog Ser 355, 297–307 (2008).
Huang, T. et al. Penguin population dynamics for the past 8500 years at Gardner Island, Vestfold Hills. Antarct Sci 21, 571–578 (2009).
Ainley, D. G. et al. Spatial and temporal variation of diet within a presumed metapopulation of Adélie Penguins. Condor 105, 95–106 (2003).
Murphy, E. J. et al. Spatial and temporal operation of the Scotia Sea ecosystem: a review of large-scale links in a krill centred food web. Phil. Trans. R. Soc. B 362, 113–148 (2007).
Nicol, S. et al. Krill (Euphausia superba) abundance and Adélie penguin (Pygoscelis adeliae) breeding performance in the waters off the Béchervaise Island colony, East Antarctica in two years with contrasting ecological conditions. Deep Sea Res Part II 55, 540–557 (2008).
Polito, M. J. et al. Integrating Stomach Content and Stable Isotope Analyses to Quantify the Diets of Pygoscelid Penguins. PLoS ONE 6(10), e26642 (2011).
Cherel, Y. Isotopic niches of emperor and Adélie penguins in Adélie Land, Antarctica. Mar. Biol. 154, 813–821 (2008).
Gillies, C. L., Stark, J. S. & Smith, S. D. A. Research article: small-scale spatial variation of δ13C and δ15N isotopes in Antarctic carbon sources and consumers. Polar Biol. 35, 813–827 (2012).
Masson, V. et al. Holocene Climate Variability in Antarctica Based on 11 Ice-Core Isotopic Records. Quatern. Res. 54, 348–358 (2000).
Denis, D. et al. Holocene productivity changes off Adélie Land (East Antarctica). Paleoceanography 24, PA3207 (2009).
McMinn, A., Heijnis, H., Harle, K. & McOrist, G. Late-Holocene climatic change recorded in sediment cores from Ellis Fjord, eastern Antarctica. Holocene 11, 291–300 (2001).
Loeb, V. et al. Effects of sea-ice extent and krill or salp dominance on the Antarctic food web. Nature 387, 897–900 (1997).
Nicol, S. et al. Ocean circulation off east Antarctica affects ecosystem structure and sea-ice extent. Nature 406, 504–507 (2000).
Rau, G. H., Ohman, M. D. & Pierrot-Bults, A. Linking nitrogen dynamics to climate variability off central California: a 51 year record based on 15N/14N in CalCOFI zooplankton. Deep-Sea Res Part II 40, 2431–2447 (2003).
Crosta, X. & Shemesh, A. Reconciling down core anticorrelation of diatom carbon and nitrogen isotopic ratios from the Southern Ocean. Paleoceanography 17, 1010 (2002).
Emslie, S. D., Coats, L. & Licht, K. A 45,000 yr record of Adélie penguins and climate change in the Ross Sea, Antarctica. Geology 35, 61–64 (2007b
Mizutani H, W. E. Nitrogen and Carbon Isotope Ratios in Seabird Rookeries and their Ecological Implications. Ecology 69, 340–349 (1988).
Emslie, S. D., Polito, M. J. & Patterson, W. P. Stable isotope analysis of ancient and modern Gentoo Penguin egg membrane supports the krill surplus hypothesis in Antarctica. Antarct Sci 25, 213–218 (2013).
Reid, K., Watkins, J. L., Croxall, J. P. & Murphy, E. J. Krill population dynamics at South Georgia 1991–1997, based on data from predators and nets. Mar Ecol Prog Ser 177, 103–114 (1999).
Acknowledgements
The authors are grateful to the Chinese Arctic and Antarctic Administration and Australian Antarctic Division for logistical support in the field work. This study was funded by NSFC (No. 40730107, 41106162), Chinese Polar Environment Comprehensive Investigation & Assessment Programmes (CHINARE2013-04-04-09, CHINARE2013-02-01-03) and the Fundamental Research Funds for Central Universities. Samples in this study were provided by the BIRDS-Sediment system. We thank two anonymous reviewers for critical comments and suggestions on an earlier version of the manuscript. Dr. Steve Emslie (University of North Carolina at Wilmington) provided insightful comments and also helped to polish the language.
Author information
Authors and Affiliations
Contributions
L.S. and T.H. conceived and designed the study. T.H. performed the experiments, N.L. performed the statistical analysis. L.S., T.H., Y.W., W.H. and N.L. analyzed the data and wrote the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Huang, T., Sun, L., Long, N. et al. Penguin tissue as a proxy for relative krill abundance in East Antarctica during the Holocene. Sci Rep 3, 2807 (2013). https://doi.org/10.1038/srep02807
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep02807
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.