Abstract
Alzheimer's disease (AD) is the most prevalent form of dementia. The amyloid beta (Aβ) peptide is the predominant candidate aetiological agent and is generated through the sequential proteolytic cleavage of the Amyloid Precursor Protein (APP) by beta (β) and gamma (γ) secretases. Since the cellular prion protein (PrPc) has been shown to regulate Aβ shedding, we investigated whether the cellular receptor for PrPc, namely the 37 kDa/67 kDa Laminin Receptor (LRP/LR) played a role in Aβ shedding. Here we show that LRP/LR co-localises with the AD relevant proteins APP, β- and γ-secretase, respectively. Antibody blockage and shRNA knock-down of LRP/LR reduces Aβ shedding, due to impediment of β-secretase activity, rather than alteration of APP, β- and γ-secretase levels. These findings indicate that LRP/LR contributes to Aβ shedding and recommend anti-LRP/LR specific antibodies and shRNAs as novel therapeutic tools for AD treatment.
Similar content being viewed by others
Introduction
Alzheimer's disease (AD) is the most prevalent form of dementia afflicting in excess of 37 million people globally1 and is associated with a multitude of genetic, environmental, epigenetic, dietary and lifestyle risk factors2,3. The neuropathological hallmarks of AD include intracellular neurofibrillary tangle formation (aggregates of hyper-phosphorylated microtubule associated protein, tau)4 and extracellular Aβ plaque deposition5. The Aβ peptide and more specifically the 42 amino acid isoform (Aβ42), is largely considered the primary disease causing agent in Alzheimer's disease (as Aβ accumulation is a pre-requisite for tau hyperphosporylation, the other AD-associated feature)6,7. Aβ is generated through the proteolytic cleavage of the amyloid precursor protein (APP) by β-secretase (BACE1 - β site APP cleavage enzyme)8 and γ-secretase (composed of 4 subunits of which the catalytic domain is composed of Presenilin (PS)9). The mechanisms underlying Aβ induction of neuronal loss (one of the key pathophysiological features of AD) are yet to be firmly established. However, it is proposed that Aβ may do so by eliciting alterations in signal transduction pathways through direct binding to cell surface receptors, such as N-Methyl-d-Aspartate (NMDA) receptors, insulin receptors or α-7 nicotinic receptors10,11. Alternatively, Aβ may alter signal transduction pathways indirectly via incorporation into lipid membranes of the plasma membrane and to a lesser extent cellular organelles11,12. This is thought to induce structural and functional alterations in lipid bound receptors and consequently results in aberrant signal transduction pathways12.
In 2007, Parkin et al. demonstrated a link between cellular prion proteins (PrPc) and the amyloidogenic processing of APP13. It was shown that PrPc mediates a decrease in Aβ shedding by regulating β-secretase cleavage of APP. In addition, PrPc was suggested to be a high affinity receptor for Aβ oligomers and vital in mediating the neurotoxic effects of Aβ14. PrPc has also been reported to play an important role in synaptic and neuronal loss15 as well as mediating toxic signalling induced by Aβ16,17.
The extracellular matrix glycoprotein, laminin, similarly exhibits an Aβ binding site, namely the IKAV peptide sequence located on the alpha (α) chain of the tri-peptide18. However, the association between laminin and Aβ is reported to inhibit fibrillogenesis18 and thereby thwart Aβ pathogenesis.
The 37 kDa/67 kDa laminin receptor (LRP/LR) (also known as LAMR, RPSA and p40) is a multifunctional protein located within the cholesterol-rich lipid raft domains of the plasma membrane, in the cytoplasm as well as in the nucleus19. Associations between the receptor and a multitude of extracellular (laminin and elastin) and intracellular (cytoskeletal proteins, histones, heparan sulfate proteoglycans (HSPGs)) components have been described and are of physiological significance both in healthy and cancerous cells20,21,22,23,24. Moreover, it has been established that LRP/LR is a high affinity receptor for laminin and both the cellular and infectious prion protein isoforms (PrPc and PrPSc, respectively)25,26,27,28 and plays an important role in the binding, receptor mediated endocytosis and propagation of these proteins29,30. As LRP/LR and Aβ share the aforementioned mutual binding partners, we proposed that LRP/LR is implicated in AD pathogenesis. However, a relationship between these proteins has as yet not been investigated.
Results
LRP/LR co-localises with APP, β- and γ-secretase on the cell surface
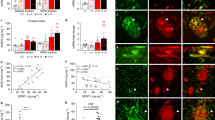
To assess whether LRP/LR and AD relevant proteins APP, β- and γ-secretase share a similar cell surface localisation, indirect immunofluorescence microscopy was employed. LRP/LR was shown to co-localise with APP (Fig. 1 and Fig. S1, a–d), β-secretase (Fig. 1 and Fig. S1, e–h), γ-secretase (Fig. 1 and Fig. S1, i–l) on the surface of non-permeabilised HEK293 (Fig. 1) and N2a cells (Fig. S1), as depicted by the yellow merged images. 2D-cytofluorograms (Fig. 1 and Fig. S1, d, h, l) reveal a yellow diagonal confirming co-localisation between the corresponding cell surface proteins. Pearson's Correlation co-efficient was employed to further confirm the observed results (Table 1). A Pearson's Correlation co-efficient of 1 is indicative of perfectly correlated proteins31. The obtained Pearson's correlation co-efficient between LRP/LR and the AD relevant proteins are all approximately within the 0.9 range (Table 1). An alternative laminin binding receptor, Very Late Antigen 6 (VLA6), employed as a negative control failed to co-localise with LRP/LR (Fig. 1 and Fig. S1, m–p, Table 1). The proximity of these proteins on the cell surface thereby suggests that an association/interaction between the receptor and AD relevant proteins is feasible.
Co-localisation of LRP/LR with the AD relevant proteins APP, β- and γ-secretase on the cell surace.
Cell surface proteins on HEK293 cells were indirectly immunolabelled to allow for detection using the Olympus IX71 Immunofluorescence Microscope and Analysis Get It Research Software. (a) APP, (e) β-secretase, (i) γ-secretase and (m) VLA6 were all indirectly labelled with Alexafluor 633, while an anti-human FITC conjugated antibody was used to label IgG1-iS18 bound to LRP/LR (b, f, j, n). The merged images between LRP/LR and relevant proteins are shown (c, g, k, o) and the corresponding 2D-cytofluorograms have been included to confirm the degree of co-localisation (d, h, l, p).
LRP/LR co-localises with APP, β- and γ-secretase within the cell
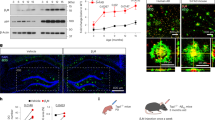
To further investigate whether LRP/LR co-localises with APP, β- and γ-secretase in subcellular locations other than the cell surface, HEK293 cells were transfected with plasmids encoding fluorescently tagged proteins and examined using confocal microscopy. APP-GFP, BACE1-GFP (β-secretase) and PS1-GFP (Presenilin 1 – the catalytic subunit of the γ-secretase) were respectively co-expressed with LRP-dsRed post transfection. The results obtained suggest that LRP/LR does not only co-localise with the AD relevant proteins on the cell surface but also intracellularly in the cytoplasm. From Fig. 2c, a greater degree of co-localisation is observed between APP (APP-GFP) and LRP/LR (LRP-dsRed) in the cytoplasm and to a lesser extent in the nucleus of the HEK293 cells as shown by the intensity of the yellow stain. Fig. 2g highlights that the co-localisation between β-secretase (BACE1-GFP) and LRP/LR (LRP-dsRed) is confined to areas of the cytoplasm, but completely lacking in the nucleus. The co-localisation between γ-secretase (PS1-GFP) and LRP/LR (LRP-dsRed) reveals a similar relationship in the cytoplasm, however, there also appears to be a high degree of co-localisation on the cell membrane as emphasized by staining seen in Fig. 2k. The presence of any co-localisation between LRP/LR and γ-secretase is completely lacking in the nucleus of the cells. GFP was used as a negative control and shows very little co-localisation with LRP/LR as is evidenced by the weak yellow signal obtained in Fig. 2o. The 2D cytofluorograms (Fig. 2d, h, l) further confirm that co-localisation does occur between LRP/LR and the AD relevant proteins APP, β- and γ-secretase, as a distinct diagonal passing through quadrant 3 is seen. The 2D cytofluorogram between GFP and LRP/LR (Fig. 2P) shows a complete lack of a diagonal and does not pass through quadrant 3, therefore no co-localisation between LRP/LR and GFP is likely to exist other than by random chance (as is seen by the weak yellow signals in Fig. 2o). From Table 1, it is evident that a positive correlation exists between LRP/LR and APP, β- and γ-secretase, as the Pearson's co-efficient values are all above 0. The value seen for GFP and LRP (−0.51) is negative and indicates that there is a low level of correlation between the cellular localisation of these 2 proteins.
Co-localisation of LRP-dsRed with APP-GFP, BACE1-GFP and PS1-GFP inside HEK293 cells.
Fluorescently tagged (a) APP-GFP, (e) BACE1-GFP, (i) PS1-GFP and (m) GFP were co-expressed with LRP-dsRed (b, f, j, n) in HEK293 cells. Images were captured using the Zeiss LSM 780 confocal microscope and analysed using Zen 2010 software. The resulting merges between LRP-dsRed and APP-GFP (c), BACE1-GFP (g), PS1-GFP (k) and GFP (o) are shown and the corresponding 2D cytofluorograms for each merge have also been included (d, h, l, p) as a measure of the degree of co-localisation.
IgG1-iS18 and shRNA treatment targeting LRP/LR significantly reduces Aβ shedding
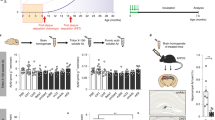
To investigate whether LRP/LR is involved in the amyloidogenic pathway and more specifically Aβ shedding into the extracellular space, cells were treated with the anti-LRP/LR specific antibody IgG1-iS1832 and anti-cluster of differentiation (CD19) antibody IgG1-HD3732 (negative control). Cellular incubation with IgG1-iS18 resulted in a significant reduction in Aβ concentration when compared to the no antibody control (Fig. 3a). Aβ levels were lowered by 47.6% in HEK293 cells (P = 0.0008) and 28.5% in SH-SY5Y cells (P = 0.0064) (Fig. 3a) after IgG1-iS18 treatment. To further assess the effects of IgG1-iS18 on Aβ shedding, a dose dependency assay was conducted using SH-SY5Y cells. Aβ shedding was significantly hampered by between 24% (for 25 μg/ml) and 43% (for 100 μg/ml) denoting that IgG1-iS18 impedes Aβ shedding in a dose-dependent fashion (Fig. 3b).
Effects of IgG1-iS18.
(a) Aβ levels in HEK293 and SH-SY5Y cells after treatment with IgG1-iS18 and IgG1-HD37 as detected by an Aβ ELISA after 18 hours of antibody incubation. Data shown (mean ± s.e.m) representative of three independent experiments (performed in triplicate) per cell line. *p < 0.05, **p < 0.01, ***p < 0.001, NS not significant; Student's t-test. (b) Aβ concentrations after SH-SY5Y cells were treated with varying doses of IgG1-iS18 for 18 hours, as determined by an Aβ ELISA. Data shown (Mean ± s.d.) comparing Aβ levels of untreated cells (0 μg/ml) and IgG1-iS18 treated cells (25–100 μg/ml), ***p < 0.0001; n = 3; one way ANOVA. (c) Flow cytometric analysis of APP, β-secretase and γ-secretase levels on the surface of HEK293 cells post treatment with IgG1-iS18 (mean ± s.d., NS not significant, n = 3, Student's t-test). (d) (i) Western blot showing sAPPβ levels from cell culture medium after SH-SY5Y cells were treated with varying concentrations (0–100 μg/ml) of IgG1-iS18 for 18 hours. Western blot band intensities from three independent experiments were quantified using Quantity One 4.6 software. Gels have been cropped for clarity and conciseness purposes and have been run under the same experimental conditions. (d) (ii) Obtained band intensities were subsequently used to determine the percentage downregulation of sAPPβ. Data shown (mean ± s.d); ***p < 0.0001; One way ANOVA.
Owing to the ability of IgG1-iS18 to decrease Aβ shedding, it may be proposed that LRP/LR mediates this process. To further confirm this role in the amyloidogenic pathway, RNA interference technology, specifically short hairpin RNA (shRNA), was employed to downregulate LRP/LR expression. shRNA1 and shRNA7 resulted in a significant 42.85% and 16.42% decrease in LRP/LR expression levels, respectively, compared to the scrambled control (shRNAscr) (Fig. 4a). This downregulation correlated to a significant 16.88% and 11.95% decrease in Aβ shedding in HEK293 cells (for shRNA1 and shRNA7 respectively) (Fig. 4b). No significant difference was observed between mock-transfected and shRNAscr control transfected HEK293 cells (Fig. S3).
Effects of LRP/LR downregulation by shRNA.
(a) HEK293 cells were transfected with LRP/LR-specific shRNA1 and shRNA7 (as well as a scrambled control, shRNAscr). 72 hours post-transfection, cells were lysed and LRP/LR levels assessed by Western blotting. β-actin was used as a loading control. Western blot band intensities from three independent experiments were quantified using Quantity One 4.6 Software. (b) The Aβ concentration of the cell culture medium of shRNA-transfected HEK293 cells was analysed using an Aβ ELISA. Data shown (Mean ± s.d.) comparing Aβ levels of shRNA1 and shRNA7 to shRNAscr, *p < 0.05, **p < 0.01; n = 3; Student's t-test. (c) Flow cytometric analysis of APP, β-secretase and γ-secretase levels on the surface of shRNA-transfected HEK293 cells. Data shown (Mean ± s.d.); n = 3; Student's t-test. (d)(i) sAPPβ levels in shRNA-transfected HEK293 cells were analysed by Western blotting. Gels have been cropped for clarity and conciseness purposes and have been run under the same experimental conditions. (d)(ii) Band intensities were extracted as mentioned previously (Fig. 3 d) and used to calculate the percentage of downregulation of sAPPβ levels.
IgG1-iS18 and LRP/LR shRNA treatments do not alter cell surface expression of APP, β- and γ-secretase
To investigate whether LRP/LR influences the amyloidogenic pathway through altering cell surface protein expression levels of the aforementioned AD relevant proteins, flow cytometric analysis of the cell surface levels of APP, β-secretase and γ-secretase was performed post-antibody (IgG1-iS18 and IgG1-HD37) treatment in HEK293 cells (Fig. 3C and Fig. S4a). Both antibody treatment and downregulation of LRP/LR by shRNA1 and 7 (Fig. 4c, Fig. S4 b) did not significantly alter HEK293 cell surface expression levels of the APP, β- and γ-secretase in comparison to controls.
sAPPβ levels are affected by LRP blockade and downregulation
In an attempt to elucidate the mechanism whereby LRP/LR influences the amyloidogenic pathway, sAPPβ levels were assessed post-antibody (Fig. 3d(i)) and shRNA treatment (Fig. 4d (i)). Upon a dose dependent administration of IgG1-iS18 to SH-SY5Y cells, a significant reduction in sAPPβ levels was observed across all antibody concentrations (Fig. 3d(ii)). Similar results were obtained for shRNA1 mediated LRP/LR downregulated HEK293 cells (Fig. 4d).
LRP/LR interacts with β-secretase
A FLAG Immunoprecipitation assay was conducted using a FLAG-tagged variant of LRP (LRP::FLAG) which had the ability to bind to anti-FLAG M2 beads. Any protein interacting with LRP::FLAG would thus remain immobilised during subsequent washing steps and would be present in the eluate of the immunoprecipitation assay. A FLAG Immunoprecipitation assay was thus performed to detect whether LRP/LR shows any interaction with β-secretase in order to further validate the co-localisation results observed between LRP/LR and β-secretase, as well as effects seen on sAPPβ shedding upon IgG1-iS18 incubation. From Fig. 5, it is evident that a distinct band is present in the eluate of the FLAG Immunoprecipitation assay (Fig. 5, lane 4) corresponding in size to the band detected in the BACE1-GFP expressing cell lysate control lane (Fig. 5, lane 3). This suggests that an interaction does exist between LRP/LR and β-secretase, as only proteins that interact with LRP would be present in the eluate. CAT (chloramphenicol acetyl transferase) transfected cell lysates were used as a negative control. CAT was not present in the immunoprecipitation assay eluate, thus showing no interaction with LRP (Fig. S5).
LRP::FLAG Immunoprecipitation Assay Analysis.
HEK293 cell lysates containing LRP::FLAG and either BACE1-GFP or CAT (Fig. S5) were subjected to Pull Down Assay analysis using Anti-FLAG M2 beads. After subsequent wash steps, eluted fractions were analysed using Immunoblotting. Lane 1 contained non-transfected (NT) cell lysate, lane 2 contained GFP transfected cell lysate, lane 3 contained BACE1-GFP transfected cell lysate and lane 4 contained the eluate from the FLAG Immunoprecipitation assay between BACE1-GFP and LRP::FLAG. Gels have been cropped for clarity and conciseness purposes and have been run under the same experimental conditions.
Discussion
The co-localisation observed between LRP/LR and the relevant AD proteins (APP, β and γ-secretase) on both HEK293 and N2a cells, as assessed by immunofluorescence microscopy, indicates that a spatial overlap occurs between the fluorescently immunolabeled proteins (Fig. 1 and Fig. S1). Although the close cellular proximity of the proteins on the cell surface is not explicitly indicative of an interaction, it does imply that an association between these proteins and LRP/LR is possible.
Confocal microscopy was further utilized to examine whether LRP/LR co-localised with APP, β- and γ-secretase in sub-cellular locations other than the cell surface. From Fig. 2, it is evident that LRP/LR also shows a high degree of co-localisation with the AD relevant proteins within the cytoplasm. LRP/LR is known to be present in the cytoplasm33 and lipid raft regions34 of the cell membrane from where it was reported to co-localise with PrPc in the early endosomes upon LRP/LR-dependent PrPc endocytosis29. With regard to APP, it is proposed that this protein is internalized from the lipid raft region of the plasma membrane into early endosomes via either clathrin35 or raft-mediated endocytosis36. β-secretase is also located in the lipid raft regions where it too is endocytosed into early endosomes37 via the GTPase Arf638. All 4 subunits of the γ-secretase have been found to be present in lipid raft regions of not only the plasma membrane39, but of the Golgi and endosomal membranes40 too. The presence of APP, β- and γ-secretase in the endosomes led to the finding that APP is preferentially cleaved by β-secretase at this site due to the lower pH within endosomes8. However, the three AD related proteins are also found in other subcellular locations, such as the Endopasmic reticulum, Golgi and trans-Golgi network; and recent studies have shown that APP cleavage can also occur at these sites41,42. This suggests a widespread distribution of APP and its cleavage proteins within various cellular structures and various internalization routes for each of the proteins involved. From our results, the intracellular distribution of APP, β- and γ-secretase is seen to be fairly widespread through the cytoplasm (Fig. 2a, e and i respectively) – due to the aforementioned cellular organelles in which they are found. From the literature it is evident that LRP/LR and the AD relevant proteins exist in similar cellular locations and from the results obtained in Fig. 2 (b, f and j), LRP/LR is indeed found to be present in very similar intracellular regions (Fig. 2 c, g and k) as these proteins. This suggests that LRP/LR could be interacting with APP, β- and γ-secretase within the cellular structures in which they are found, or alternatively, could potentially be involved in the regulation of APP processing.
LRP/LR blockage by IgG1-iS18 was seen to effectively impede Aβ shedding. This was further affirmed by shRNA mediated downregulation of the receptor. These results taken together suggest that LRP/LR plays a pivotal role in the amyloidogenic processing of APP.
Interestingly, LRP blockage did not result in modulation of cell surface levels of AD relevant proteins, thereby inferring that the influence of LRP/LR may rather be as a result of protein interactions.
sAPPβ is the initial cleavage product of APP by β-secretase and is released into the extracellular space. The administration of IgG1-iS18 at increasing concentrations resulted in a dose dependent decrease in sAPPβ levels, suggesting that blocking LRP/LR impedes β-secretase activity. Similar results were seen when LRP/LR was downregulated, thus further corroborating the possibility of an interaction between these two proteins. These results implicate LRP/LR in the amyloidogenic process, specifically via promoting β-secretase activity. Since LRP/LR and β-secretase co-localize and sAPPβ shedding is significantly impeded by IgG1-iS18 and shRNA treatment, we proposed that an interaction between β-secretase and LRP/LR exists. This was verified upon performing a FLAG Immunoprecipitation assay utilizing cell lysates of cells expressing LRP::FLAG and BACE1-GFP (Fig. 5). BACE1-GFP was detected in the eluate of the immunoprecipitation and since only proteins binding to the LRP::FLAG protein would be present in this fraction, this strongly suggests that an interaction between LRP/LR and β-secretase does exist. However, as crude cell lysates expressing LRP::FLAG and BACE1-GFP were utilised for the assay, it is also possible that any interaction that exists between these two proteins may be an indirect one, mediated by another protein present in the cell lysate.
Efforts to develop effective therapies for AD have to take into consideration that targeting the secretases may cause off target effects, such as a disruption of BACE1 processing of CHL1 which is required for axonal guidance43. Here we imply that the role of β-secretase in the amyloidogenic pathway is augmented by LRP/LR, as the blockage of LRP/LR reduces the shedding of both sAPPβ and Aβ and LRP/LR is seen to interact with β-secretase. The effects of hindering the interaction between β-secretase and LRP/LR would thus have to be examined in detail in future studies, to test for the effects it may have on the other physiological roles in which β-secretase is involved. Cell viability assays, however, have been performed using IgG1-iS18 on HEK293 cells and no significant reduction in cell viability was observed44, thus suggesting whatever interaction the antibody may be blocking between β-secretase and LRP/LR is not detrimental to the cells.
The result of the FLAG Immunoprecipitation assay further confirmed the initial findings suggested by the co-localisation (Fig. 1) and confocal data (Fig. 2) that an interaction is likely to occur between LRP/LR and β-secretase both due to their proximity on the cell surface and within the cell. Thus it further corroborates the possibility that interactions may also occur between LRP/LR and APP and/or γ-secretase.
In conclusion, we have identified a novel role for LRP/LR in AD and more specifically in enhancing β-secretase cleavage of APP. Blockage and downregulation of LRP/LR resulted in a significant reduction in Aβ levels suggesting that anti-LRP/LR specific antibodies and shRNAs could be used as possible alternative therapeutic tools for AD treatment.
Methods
Tissue culture
HEK293 cells were grown in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% Fetal Calf Serum (FCS) and 1% Penicillin/Streptomycin (P/S) solution. N2a cells were grown in Optimem with 10% FCS and 1% P/S. SH-SY5Y cells were cultured in 1:1 EMEM:F12 media supplemented with 15% FCS, 1% Non-Essential Amino Acids, 1% L-Glutamine and 1% P/S. Cells were incubated at 37°C in a humidified 5% CO2 atmosphere.
Immunofluorescence microscopy
HEK239 and N2a cells were seeded onto sterile microscope coverslips and incubated for 24 hours (37°C, 5% CO2) allowing the cells to reach 50–70% confluency. The cells were fixed with 4% Paraformaldehyde (10 minutes, room temperature). The coverslips were rinsed 3 times in PBS and blocked in 0.5%PBS-BSA solution for 5–10 minutes. Following an additional wash in PBS, the coverslips were placed with the cells facing upwards on a microscope slide. 100 μl of primary antibody solution (diluted in 0.5%PBS-BSA) containing 1:150 IgG1-iS18 and either anti-APP (rabbit polyclonal IgG) (Abcam), anti-BACE (M-83) (rabbit polyclonal IgG) (Santa Cruz Biotechnology), anti-PEN-2 (FL-101) (rabbit polyclonal IgG) (Santa Cruz Biotechnology) or anti-very late antigen-6 (VLA6) CD49-f (rabbit monoclonal IgG) (Immunotech) was added to the cells. These slides were then incubated overnight at 4°C in moist containers. Cover slips were then rinsed 3 times in 0.5% PBS-BSA and placed on clean slides. The cells were treated with 100 μl of a secondary antibody solution containing goat anti-human FITC (Cell Lab) (1:350–1:400) (specific for IgG1-iS18) and Alexa Fluor 633 goat anti-rabbit IgG (Invitrogen) (specific for the anti-APP, anti-BACE, anti PEN-2 or anti-VLA6 primary antibodies). After an hour's incubation in the dark, the coverslips were washed twice in 0.5% PBS-BSA and once in PBS and were subsequently mounted onto clean microscope slides using 75 μl Fluoromount (Sigma Aldrich). These slides were incubated at room temperature in the dark for 1–2 hours to allow the mounting medium to set.
Images were acquired at room temperature with 60× magnification using the Olympus IX71 Immunofluorescence Microscope and Olympus XM10 greyscale camera. analySIS Research Image Processing Software was used to capture the images and they were subsequently analysed (and 2D cytofluorograms were constructed) using CellSens Dimension Software.
Confocal microscopy
HEK 293 cells were transfected with plasmids encoding for LRP-dsRed and APP-GFP, BACE1-GFP or PS1-GFP respectively. 24 hours post transfection, coverslips were washed with PBS 3 times and then fixed with 4% Paraformaldehyde for 15 minutes. Coverslips were subsequently washed in PBS and mounted cell side down onto clean microscope slides using 75 μl Fluoromount (Sigma Aldrich). These slides were incubated at room temperature for 1–2 hours in the dark to allow the mounting medium to set.
Slides were viewed using the Zeiss LSM 780 confocal microscope and captured using the AxioCam MRm camera. Images were subsequently analysed using Zen 2010 imaging software.
Antibody treatment
HEK293 cells were grown to 50–70% confluency. The tissue culture medium was aspirated and replaced with media containing 50 μg/ml IgG1-iS1832, 50 μg/ml IgG1-HD3732, or no antibody. Each of the respective treatments were performed in triplicate. The cells were subsequently incubated at 37°C, 5%CO2 for 18 hours.
LRP/LR target sequences and structure of shRNA1 and shRNA7
The complete shRNA expression cassettes were designed with the guide strand on the 3′arm, a poly T termination signal and to include a full H1 RNA polymerase III promoter sequence. To prepare the shRNA cassettes, the H1 RNA Pol III promoter was used as a template in a nested PCR, whereby the sequences corresponding to the shRNAs were incorporated into two reverse primers (one for the primary PCR and one for the secondary PCR). The same forward primer, which is complementary to the start of the H1 promoter, was used in both. The PCR products coding for the shRNA expression constructs were sub-cloned into the pTZ57R/T vector (Fermentas). A scrambled shRNA (shRNAscr) that does not target any gene product was used as a negative control. See figure S2 for LRP/LR target sequence and shRNA 1 and shRNA7 structure.
Cell transfection
The construction of pCIneo-moLRP::FLAG45 and pLRP-dsRed46 have been described previously, while pEGFPN1-APP77037 and pEGFPN1-BACE147 were generous gifts from Dr. Bradley T Hyman. pEGFP-PS148 was a kind gift from Dr. Oskana Berezovska. pEGFP-N1 (Clonetech) and pCDNA3/CAT (Invitrogen) were used as control plasmids. For confocal microscopy, HEK 293 cells were seeded onto coverslips in 6 well plates and incubated overnight. The following day, when cells were 30–50% confluent, calcium phosphate transfection was carried out using 86 μl of 1 × HBS, 5.1 μg of total plasmid DNA (i.e. 2.55 μg of each plasmid was used in co-transfections) and 5.1 μl of 2.5 M CaCl2. Cells were incubated for 24 hours and then used for confocal microscopy. HEK293 cells were transfected with LRP/LR shRNA 1 and 7 according to the manufacturer's instructions, using TransIT-LT1 Transfection Reagent (Mirus). Transfected cells were incubated for 72 hours prior to analysis. For FLAG Immunoprecipitation assays, HEK293 cells were seeded into 60 mm tissue culture plates and grown to 30–50% confluency overnight. Calcium phosphate transfection was carried out using 186 μl 1 × HBS, 11 μg of either pCIneo-moLRP::FLAG, pEGFPN1-BACE1 or pCDNA3/CAT and 11 μl 2.5 M CaCl2. Cells were incubated for 72 hours prior to analysis.
Amyloid beta enzyme-linked immunosorbent assay (ELISA)
The amyloid beta (Aβ) assay was performed using the Human Amyloid β (1 − x) Assay kit (Immuno-Biological Laboratories Co.,Ltd) – a solid phase ELISA. This assay was performed as per manufacturer's instructions using the tissue culture media after antibody and shRNA treatment (see above).
Flow cytometry
Cell lines were subjected to flow cytometric analysis following treatment with IgG1-iS18 or shRNA1 and 7. Cells were fixed using 4% paraformaldehyde (10 minutes,4°C), centrifuged at 1500 g (10 minutes, 4°C) and resuspended in 1 ml IsoFlow™ EPICS™ Sheath Fluid (Beckman Coulter). Half of the volume of each sample was treated with 30 ug/ml of either anti-APP (rabbit polyclonal IgG) (Abcam), anti-BACE (M-83) (rabbit polyclonal IgG) (Santa Cruz Biotechnology) or anti-PEN-2 (FL-101) (rabbit polyclonal IgG) (Santa Cruz Biotechnology) while remaining sample volume was incubated in IsoFlow™ EPICS™ Sheath Fluid (Beckman Coulter). Samples were incubated for 1 hour at room temperature and subsequently washed three times in IsoFlow™ EPICS™ Sheath Fluid (Beckman Coulter) (1700 g, 4°C). All samples were treated with 20 ug/ml of the corresponding goat anti-rabbit FITC coupled secondary antibody (Cell Lab) and incubated for 1 hour at room temperature. The samples were washed a further three times (as above) and the cell suspensions were transferred to flow cytometry tubes. The samples were analysed using Coulter EPICS XL-MCL (for antibody treated samples) or the BD Accuri C6 (for shRNA treated samples) and 10 000–50 000 events recorded.
Western blotting
LRP/LR levels were determined by immunoblotting using IgG1-iS18 (1:10 000) and goat anti-human horseradish peroxidise (HRP) (1:10 000) (Cell Lab). sAPPβ levels were assessed by immunoblotting using sAPPβ-Wild type Rabbit IgG (1:1000) (Immuno-Biological Laboratories Co., Ltd) and goat anti-rabbit HRP (1:10 000) (Cell Lab). FLAG Immunoprecipitation assay eluate samples were analysed using either anti-FLAG (mouse monoclonal IgG) (1:5000) (Sigma-Aldrich), anti-BACE1 (rabbit polyclonal IgG fraction) (1:3000) (Abcam) or anti-Chloramphenicol Acetyl Transferase (rabbit IgG fraction) (1:5000)(Sigma-Aldrich). Anti-mouse HRP (1:5000) (Sigma-Aldrich) or anti-rabbit HRP (1:5000) (Sigma-Aldrich) were used as secondary antibodies. Immunodetection was carried out using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific) and X-ray film.
FLAG immunoprecipitation assay
HEK293 cells were lysed 72 hours post-transfection with pCIneo-moLRP::FLAG, pEGFPN1-BACE1 or pCDNA3/CAT. Cell lysates expressing LRP::FLAG were then mixed with those containing either BACE1-GFP or CAT. These cell lysates were then subjected to a FLAG Immunoprecipitation Kit (Sigma-Aldrich) analysis according to the manufacturer's instructions. Eluted samples were analysed via Immunoblotting.
References
Mount, C. & Downton, C. Alzheimer disease: progress or profit? Nat Med 12, 780–784 (2006).
Maloney, B. & Lahiri, D. K. The Alzheimer's amyloid beta-peptide (Abeta) binds a specific DNA Abeta-interacting domain (AbetaID) in the APP, BACE1 and APOE promoters in a sequence-specific manner: characterizing a new regulatory motif. Gene 488, 1–12 (2011).
Holtzman, D. M., Herz, J. & Bu, G. Apolipoprotein e and apolipoprotein e receptors: normal biology and roles in Alzheimer disease. Cold Spring Harb Perspect Med 2, a006312, http://dx.doi.org/10.1101/cshperspect.a006312 (2012).
Avila, J. Tau phosphorylation and aggregation in Alzheimer's disease pathology. FEBS Lett 580, 2922–2927 (2006).
Gonsalves, D., Jovanovic, K., Da Costa Dias, B. & Weiss, S. F. Global Alzheimer Research Summit: Basic and clinical research: Present and future Alzheimer research. Prion 6, 7–10 (2012).
Busciglio, J., Lorenzo, A., Yeh, J. & Yankner, B. A. beta-amyloid fibrils induce tau phosphorylation and loss of microtubule binding. Neuron 14, 879–888 (1995).
Ardini, E. et al. Co-regulation and physical association of the 67-kDa monomeric laminin receptor and the alpha6beta4 integrin. J Biol Chem 272, 2342–2345 (1997).
Vassar, R. et al. Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286, 735–741 (1999).
Edbauer, D. et al. Reconstitution of gamma-secretase activity. Nat Cell Biol 5, 486–488, http://dx.doi.org/10.1038/ncb960 (2003).
Verdier, Y. & Penke, B. Binding sites of amyloid beta-peptide in cell plasma membrane and implications for Alzheimer's disease. Curr Protein Pept Sci 5, 19–31 (2004).
Da Costa Dias, B., Jovanovic, K., Gonsalves, D. & Weiss, S. F. Structural and mechanistic commonalities of amyloid-beta and the prion protein. Prion 5, 126–137 (2011).
Sepulveda, F. J., Parodi, J., Peoples, R. W., Opazo, C. & Aguayo, L. G. Synaptotoxicity of Alzheimer beta amyloid can be explained by its membrane perforating property. PLoS One 5, e11820, http://dx.doi.org/10.1371/journal.pone.0011820 (2010).
Parkin, E. T. et al. Cellular prion protein regulates beta-secretase cleavage of the Alzheimer's amyloid precursor protein. Proc Natl Acad Sci U S A 104, 11062–11067 (2007).
Lauren, J., Gimbel, D. A., Nygaard, H. B., Gilbert, J. W. & Strittmatter, S. M. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature 457, 1128–1132 (2009).
Kudo, W. et al. Cellular prion protein is essential for oligomeric amyloid-beta-induced neuronal cell death. Hum Mol Genet 21, 1138–1144 (2012).
Resenberger, U. K., Winklhofer, K. F. & Tatzelt, J. Cellular Prion Protein Mediates Toxic Signaling of Amyloid Beta. Neurodegener Dis 10, 298–300 (2012).
Um, J. W. et al. Alzheimer amyloid-beta oligomer bound to postsynaptic prion protein activates Fyn to impair neurons. Nat Neurosci 15, 1227–1235 (2012).
Castillo, G. M. et al. Laminin inhibition of beta-amyloid protein (Abeta) fibrillogenesis and identification of an Abeta binding site localized to the globular domain repeats on the laminin a chain. J Neurosci Res 62, 451–462 (2000).
Mbazima, V., Da Costa Dias, B., Omar, A., Jovanovic, K. & Weiss, S. Interactions between PrPc and other ligands with the 37-kDa/67-kDa laminin receptor. Frontiers in Bioscience 15, 1150–1163 (2010).
Moodley, K. & Weiss, S. F. Downregulation of the non-integrin laminin receptor reduces cellular viability by inducing apoptosis in lung and cervical cancer cells. PLoS One 8, e57409, http://dx.doi.org/10.1371/journal.pone.0057409 PONE-D-12-36482 (2013).
Omar, A. et al. Patented biological approaches for the therapeutic modulation of the 37 kDa/67 kDa laminin receptor. Expert Opin Ther Pat 21, 35–53, http://dx.doi.org/10.1517/13543776.2011.539203 (2011).
Khusal, R. et al. In vitro inhibition of angiogenesis by antibodies directed against the 37 kDa/67 kDa laminin receptor. PLoS One 8, e58888, http://dx.doi.org/10.1371/journal.pone.0058888 (2013).
Khumalo, T. et al. Adhesion and Invasion of Breast and Oesophageal Cancer Cells Are Impeded by Anti-LRP/LR-Specific Antibody IgG1-iS18. PLoS One 8, e66297, http://dx.doi.org/10.1371/journal.pone.0066297 PONE-D-13-07741 (2013).
Omar, A., Reusch, U., Knackmuss, S., Little, M. & Weiss, S. F. Anti-LRP/LR-specific antibody IgG1-iS18 significantly reduces adhesion and invasion of metastatic lung, cervix, colon and prostate cancer cells. J Mol Biol 419, 102–109 (2012).
Gauczynski, S. et al. The 37-kDa/67-kDa laminin receptor acts as the cell-surface receptor for the cellular prion protein. EMBO J 20, 5863–5875, http://dx.doi.org/10.1093/emboj/20.21.5863 (2001).
Gauczynski, S. et al. The 37-kDa/67-kDa laminin receptor acts as a receptor for infectious prions and is inhibited by polysulfated glycanes. J Infect Dis 194, 702–709 (2006).
Rieger, R., Edenhofer, F., Lasmezas, C. I. & Weiss, S. The human 37-kDa laminin receptor precursor interacts with the prion protein in eukaryotic cells. Nat Med 3, 1383–1388 (1997).
Hundt, C. et al. Identification of interaction domains of the prion protein with its 37-kDa/67-kDa laminin receptor. EMBO J 20, 5876–5886, http://dx.doi.org/10.1093/emboj/20.21.5876 (2001).
Morel, E. et al. Bovine prion is endocytosed by human enterocytes via the 37 kDa/67 kDa laminin receptor. Am J Pathol 167, 1033–1042 [pii] (2005).
Leucht, C. et al. The 37 kDa/67 kDa laminin receptor is required for PrP(Sc) propagation in scrapie-infected neuronal cells. EMBO Rep 4, 290–295, http://dx.doi.org/10.1038/sj.embor.embor768 (2003).
Dunn, K. W., Kamocka, M. M. & McDonald, J. H. A practical guide to evaluating colocalization in biological microscopy. Am J Physiol Cell Physiol 300, C723–742 (2011).
Zuber, C. et al. Invasion of tumorigenic HT1080 cells is impeded by blocking or downregulating the 37-kDa/67-kDa laminin receptor. J Mol Biol 378, 530–539 (2008).
Romanov, V., Sobel, M. E., pinto da Silva, P., Menard, S. & Castronovo, V. Cell localization and redistribution of the 67 kD laminin receptor and alpha 6 beta 1 integrin subunits in response to laminin stimulation: an immunogold electron microscopy study. Cell Adhes Commun 2, 201–209 (1994).
Patra, S. K., Rizzi, F., Silva, A., Rugina, D. O. & Bettuzzi, S. Molecular targets of (−)-epigallocatechin-3-gallate (EGCG): specificity and interaction with membrane lipid rafts. J Physiol Pharmacol 59 Suppl 9, 217–235 (2008).
Schneider, A. et al. Flotillin-dependent clustering of the amyloid precursor protein regulates its endocytosis and amyloidogenic processing in neurons. J Neurosci 28, 2874–2882 (2008).
Ehehalt, R., Keller, P., Haass, C., Thiele, C. & Simons, K. Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J Cell Biol 160, 113–123, http://dx.doi.org/10.1083/jcb.200207113 (2003).
Kinoshita, A. et al. Demonstration by FRET of BACE interaction with the amyloid precursor protein at the cell surface and in early endosomes. J Cell Sci 116, 3339–3346 (2003).
Sannerud, R. et al. ADP ribosylation factor 6 (ARF6) controls amyloid precursor protein (APP) processing by mediating the endosomal sorting of BACE1. Proc Natl Acad Sci U S A 108, E559–568 (2011).
Chyung, J. H., Raper, D. M. & Selkoe, D. J. Gamma-secretase exists on the plasma membrane as an intact complex that accepts substrates and effects intramembrane cleavage. J Biol Chem 280, 4383–4392 (2005).
Vetrivel, K. S. et al. Association of gamma-secretase with lipid rafts in post-Golgi and endosome membranes. J Biol Chem 279, 44945–44954, http://dx.doi.org/10.1074/jbc.M407986200 (2004).
Griffiths, H. H. et al. Prion protein interacts with BACE1 protein and differentially regulates its activity toward wild type and Swedish mutant amyloid precursor protein. J Biol Chem 286, 33489–33500 (2011).
Pasternak, S. H., Callahan, J. W. & Mahuran, D. J. The role of the endosomal/lysosomal system in amyloid-beta production and the pathophysiology of Alzheimer's disease: reexamining the spatial paradox from a lysosomal perspective. J Alzheimers Dis 6, 53–65 (2004).
Hitt, B. et al. beta-Site amyloid precursor protein (APP)-cleaving enzyme 1 (BACE1)-deficient mice exhibit a close homolog of L1 (CHL1) loss-of-function phenotype involving axon guidance defects. J Biol Chem 287, 38408–38425 (2012).
Da Costa Dias, B. et al. Anti-LRP/LR specific antibody IgG1-iS18 and knock-down of LRP/LR by shRNAs rescue cells from Ab42 induced cytotoxicity. Sci. Rep. 3, 2702; http://dx.doi.org/10.1038/srep02702 (2013).
Vana, K. & Weiss, S. A trans-dominant negative 37 kDa/67 kDa laminin receptor mutant impairs PrP(Sc) propagation in scrapie-infected neuronal cells. J Mol Biol 358, 57–66 (2006).
Nikles, D. et al. Subcellular localization of prion proteins and the 37 kDa/67 kDa laminin receptor fused to fluorescent proteins. Biochim Biophys Acta 1782, 335–340 (2008).
von Arnim, C. A. et al. Demonstration of BACE (beta-secretase) phosphorylation and its interaction with GGA1 in cells by fluorescence-lifetime imaging microscopy. J Cell Sci 117, 5437–5445 (2004).
Uemura, K. et al. Allosteric modulation of PS1/gamma-secretase conformation correlates with amyloid beta(42/40) ratio. PLoS One 4, e7893, http://dx.doi.org/10.1371/journal.pone.0007893 (2009).
Acknowledgements
This work is based upon research supported by the National Research Foundation (NRF), the Republic of South Africa (RSA). Any opinions, findings and conclusions or recommendations expressed in this material are those of the author(s) and therefore, the National Research Foundation does not accept any liability in this regard thereto. We thank Dr. Bradley T. Hyman (Harvard Medical School, Massachusetts, USA) for his generous gift of pEGFPN1-APP770 and pEGFPN1-BACE1. We also thank Dr. Oksana Berezovska (Massachusetts General Hospital, Massachusetts, USA) for his kind gift of pEGFP-PS1.
Author information
Authors and Affiliations
Contributions
S.F.T.W. conceived and directed the project. K.J. and D.G. performed experiments. U.R., S.K., M.L. produced IgG1-iS18 and IgG1-HD37 antibodies. M.W. designed and assisted in production of shRNA; D.G. and K.M. produced the shRNA. C.P. assisted with both immunofluorescence and confocal micrsocopy. K.J., D.G., B.D.C.D. wrote manuscript and S.F.T.W. edited the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Jovanovic, K., Gonsalves, D., Da Costa Dias, B. et al. Anti-LRP/LR specific antibodies and shRNAs impede amyloid beta shedding in Alzheimer's disease. Sci Rep 3, 2699 (2013). https://doi.org/10.1038/srep02699
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep02699
This article is cited by
-
Knock-down of LRP/LR promotes apoptosis in early and late stage colorectal carcinoma cells via caspase activation
BMC Cancer (2018)
-
A highly sensitive plasma-based amyloid-β detection system through medium-changing and noise cancellation system for early diagnosis of the Alzheimer’s disease
Scientific Reports (2017)
-
The 37/67kDa laminin receptor (LR) inhibitor, NSC47924, affects 37/67kDa LR cell surface localization and interaction with the cellular prion protein
Scientific Reports (2016)
-
Correlations of amyloid-β concentrations between CSF and plasma in acute Alzheimer mouse model
Scientific Reports (2014)
-
The 37kDa/67kDa Laminin Receptor acts as a receptor for Aβ42 internalization
Scientific Reports (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.