Abstract
Cordyceps sinensis is a medicinal mushroom used for centuries in Asian countries as a health supplement and tonic. Hirsutella sinensis—the anamorphic, mycelial form of C. sinensis—possesses similar properties and is increasingly used as a health supplement. Recently, C. sinensis extracts were shown to inhibit the production of the pro-inflammatory cytokine IL-1β in lipopolysaccharide-treated macrophages. However, the molecular mechanism underlying this process has remained unclear. In addition, whether H. sinensis mycelium (HSM) extracts also inhibit the production of IL-1β has not been investigated. In the present study, the HSM extract suppresses IL-1β and IL-18 secretion and ATP-induced activation of caspase-1. Notably, we observed that HSM not only reduced expression of the inflammasome component NLRP1 and the P2X7R but also reduced the activation of caspase-4 and ATP-induced ROS production. These findings reveal that the HSM extract has anti-inflammatory properties attributed to its ability to inhibit both canonical and non-canonical inflammasomes.
Similar content being viewed by others
Introduction
Medicinal mushrooms have been used for centuries in Asia as folk medicine and natural health tonics1,2. Mushrooms like Cordyceps sinensis, Ganoderma lucidum and Agaricus blazei Murrill have been used for various human conditions, including autoimmune disease, cancer, chronic inflammation, fatigue and type II diabetes. Recent research has shown that these mushrooms produce antiviral, anticancer, anti-inflammatory and immunomodulatory effects on cultured cells and laboratory animals. Current research efforts are directed towards identifying the compounds responsible for mediating these biological effects, with polysaccharides and nucleosides appearing as major candidates1,2.
C. sinensis (also termed Ophiocordyceps sinensis) is an ascomycete fungus that possesses a peculiar mode of growth characterized by two main stages; the first stage is characterized by the fungus infecting underground caterpillar larvae in the winter, whereas the second stage is associated with the production of a fruiting body that protrudes from the dead caterpillar's head and grows above the ground during the summer3,4,5,6,7. For this reason, C. sinensis is known as the “caterpillar fungus” or “dong-chong-xia-cao” (literally “winter worm, summer grass” in Chinese)6,7,8. The growth of this natural fungus is also unusual due to the fact that it is limited to the Tibetan plateau and southwestern China and it usually grows at or even below the relatively low temperature of 18°C4,5,9. Recent studies indicate that C. sinensis has a wide range of biological activities, including anti-tumor10,11, immunomodulatory12,13, anti-inflammatory14,15, anti-oxidant16,17, anti-infection18 and anti-aging properties19.
Due to the rarity of natural C. sinensis, other means of producing this fungus have been investigated. The identity of the anamorph of C. sinensis has been a topic of considerable controversy in the past20. Hirsutella sinensis, which today is widely accepted as the true anamorphic, mycelial stage of natural C. sinensis20, is amenable to culture in vitro and is increasingly used as a health supplement. Studies of the pharmacological properties of HSM have shown that it possesses biological activities similar to that of the wild mushroom. For instance, these activities include reduction of drug-induced leucopenia following kidney transplantation, amelioration of radiation-induced toxicity and stimulation of immune cells in vivo21,22. Earlier, we demonstrated that HSM prolongs survival and decreases symptom severity in a murine model of the systemic autoimmune disease, lupus erythematosus23. However, the mechanism underlying the immunosuppressive effects of HSM is still unclear.
Methanol extracts of natural C. sinensis have been shown to suppress bronchoalveolar lavage fluid (BALF) cell proliferation and to reduce IL-1β, IL-6, IL-8, IL-10 and tumor necrosis factor (TNF)-α production in LPS-activated BALF cell cultures24. Li et al. reported that C. sinensis water extracts reduce the production of the pro-inflammatory cytokines IL-1β, IL-6, TNF-α and IL-12p70 in LPS-activated dendritic cells25. Nonetheless, whether HSM possesses similar activities has not been investigated.
Macrophages are differentiated immune cells that originate as blood monocytes and are found in tissues throughout the body. These immune cells play an essential role during initiation and propagation of inflammatory responses by producing pro-inflammatory cytokines such as IL-1β, IL-18 and TNF-α, as well as other inflammatory mediators like nitric oxide and prostaglandins26,27,28. IL-1β and IL-18, which are members of the IL-1 cytokine superfamily, promote a variety of innate immune processes associated with infection, inflammation and autoimmunity29,30. IL-1β participates in the generation of systemic and local immune responses against various strains of pathogens and has been implicated in the pathogenesis of inflammatory diseases, such as gout, asthma, inflammatory bowel diseases, rheumatoid arthritis and atherosclerosis31,32,33. IL-18 also plays a critical role in the execution of anti-microbial and anti-viral immunity and this cytokine has been associated with severe inflammatory disorders, such as rheumatoid arthritis, Crohn's disease, psoriasis, lupus, sarcoidosis and multiple sclerosis34,35.
The pro-inflammatory cytokines, IL-1β and IL-18, are synthesized as inactive precursors (i.e., pro-IL-1β and pro-IL-18) and accumulate within the cytosolic compartment of monocytes and macrophages exposed to or “primed” with pathogen-associated molecular patterns (PAMPs) like the bacterial endotoxin LPS36. However, LPS by itself is usually insufficient to trigger IL-1β and IL-18 secretion from macrophages unless danger-associated molecular patterns (DAMPs) provide the second signal responsible for the activation of the inflammasome complex, activation of the protease caspase-1, processing of pro-IL-1β and pro-IL-18 and release of the mature cytokines from the cells37,38,39.
Extracellular adenosine 5′-triphosphate (ATP) acts as a danger signal released from injured cells during tissue damage and inflammation; it initiates inflammation and further amplifies and sustains cell-mediated immunity through P2 receptor-mediated purinergic signaling40,41. Binding of ATP to the P2X7 receptor (P2X7R) in primed monocytes and macrophages leads to inflammasome activation and secretion of pro-inflammatory cytokines IL-1β and IL-1842.
Inflammasomes represent a group of cytoplasmic multiprotein complexes whose assembly leads to activation of the cysteine protease caspase-1, which promotes the proteolytic processing of the immature forms of IL-1β and IL-1843. The inflammasome complex is typically formed by three components consisting of a nucleotide binding and oligomerization domain (NOD)-like receptor (NLR), the ASC adaptor protein (for apoptosis-associated speck-like protein containing a caspase recruitment domain) and pro-caspase-1. Upon activation, oligomerized NLRs interact with ASC, which in turn recruits and activates caspase-1 and leads to cleavage and activation of pro-IL-1β and pro-IL-1839. The NLRP1 (nacht, leucine-rich repeat and pyrin domain containing domain-1; also known as NALP1, NAC, CARD7, DEFCAP, or CLR17.1) and NLRP3 (also known as NALP3, cryopyrin, CIAS1, or PYPAF1) inflammasomes are two of the best-characterized canonical inflammasomes described so far. A large number of stimuli have been shown to trigger activation of the NLRP3 inflammasome, including ATP, monosodium urate crystals, cholesterol crystals, UVB irradiation, pathogen-derived nucleic acids, silica, asbestos and amyloid-β44,45,46,47,48,49,50,51. LPS and muramyl dipeptide (MDP) along with ATP have been reported to induce NLRP1 inflammasome assembly, caspase-1 activation and cleavage of pro-IL-1β into its active form52,53.
More recently, non-canonical inflammasomes containing murine caspase-11 have also been reported54,55. Caspase-11 does not exist in humans, but is functionally equivalent to caspase-4 and caspase-5, which also modulate inflammasome activity56,57.
C. sinensis extracts were previously shown to inhibit the production of IL-1β in LPS-stimulated macrophages. However, the molecular mechanism responsible for this inhibition was not characterized and the possibility that IL-18 secretion may also be affected was not investigated. The main objective of the present study was to determine whether ethanol extracts of HSM have an inhibitory effect on the production of IL-1β and IL-18 in LPS-primed human macrophages. In addition, we examined whether the HSM ethanol extract can modulate inflammasome activation in macrophages. We demonstrate that HSM ethanol extract suppresses IL-1β and IL-18 secretion. The reduction of IL-1β and IL-18 production is associated with down-regulation of NLRP1, a component of one of the canonical inflammasomes. HSM also inhibits the transcription and activation of both caspase-1 and caspase-4, the latter being associated with non-canonical inflammasomes. Furthermore, ATP-induced ROS generation and P2X7R activation are suppressed by HSM.
Results
Absence of toxicity of the HSM ethanol extract on human macrophages
Whether ethanol extracts of HSM have cytotoxic effects on human cells has not been studied. Therefore, we first determined the effects of the HSM extract on the viability of THP-1 macrophages using the MTT assay. Treatment of the cells with either 1 or 2% (v/v) of the HSM ethanol extract for 24 h did not affect cell viability, compared with HSM-untreated control cells ( Figure 1 ). However, cell viability was significantly decreased when the cells were incubated with 5% of HSM ethanol extract for the same period of time ( Figure 1 ). Based on these results, we used the HSM extract at a concentration of 1 or 2% in subsequent experiments.
Absence of toxicity due to HSM treatment of human macrophages.
Cells were treated with 1 to 5% of HSM ethanol extract for 24 h and cell viability was measured by the MTT assay, as described in Materials and Methods. Data are presented as means ± SE of three experiments preformed in duplicate. *P < 0.01 versus HSM-untreated control cells.
HSM extract reduces ATP-induced IL-1β and IL-18 secretion in LPS-primed macrophages
We examined whether the HSM extract affects IL-1β and IL-18 gene expression in THP-1 macrophages. We first pre-treated the macrophages with the HSM ethanol extract (1 or 2%) for 20 h, then with LPS (0.5 μg/ml) for 3 h to induce cytokine expression and finally with ATP (5 mM) for 1 h to activate the cells and induce secretion of IL-1β and IL-18. RT-PCR and quantitative real-time PCR analyses showed that HSM pre-treatment increased the mRNA expression levels of IL-1β and IL-18 in a dose-dependent manner ( Figure 2a, 2b , 3c and 3d ).
Effects of HSM on IL-1β gene expression and secretion in human macrophages.
Cells were pre-treated with either 1 or 2% of HSM extract for 20 h, followed by treatment with LPS (0.5 μg/ml) for 3 h and with ATP (5 mM) for 1 h. (a) The mRNA expression levels of IL-1β were determined by RT-PCR analysis. (b) IL-1β mRNAs were quantified using real-time PCR. β-actin gene expression was used for normalization. The results are expressed as fold changes, considering one as the value of untreated cells. (c) The amount of IL-1β in cell culture supernatants was detected by ELISA. (d) The presence of IL-1β in cell lysates and cell culture supernatants were analyzed by Western blot analysis. Data are presented as means ± SE of three experiments performed in duplicate. #P < 0.01 versus untreated cells. *P < 0.01 versus HSM-untreated (ethanol-treated) control cells. †P < 0.05 versus HSM (1%) treated cells.
Effects of HSM on IL-18 gene expression and secretion in human macrophages.
Cells were pre-treated with various concentrations (1 or 2%) of HSM extract for 20 h, followed by treatment with LPS (0.5 μg/ml) for 3 h and ATP (5 mM) for 1 h. (a) The mRNA expression levels of IL-18 were determined by RT-PCR analysis. (b) IL-18 mRNAs were quantified using real-time PCR. β-actin gene expression was used for normalization. The results are expressed as fold changes, considering one as the value of untreated cells. (c) The amount of IL-18 in cell culture supernatants was detected by ELISA. (d) The presence of IL-18 in cell lysates and cell culture supernatants were analyzed by Western blot analysis. Data are presented as means ± SE of three experiments performed in duplicate. #P < 0.01 versus untreated cells. *P < 0.01 versus HSM-untreated control (ethanol) cells. †P < 0.05 versus HSM (1%) treated cells.
We then determined the concentrations of secreted IL-1β and IL-18 proteins in the cell culture supernatants of the same ATP-activated macrophages. ELISA and Western blot analyses revealed that pre-treatment of the cells with HSM significantly reduced the secretion of IL-1β and IL-18 in a dose-dependent manner ( Figure 2c, 2d , 3c and 3d ). These findings indicate that the HSM extract stimulates expression of the cytokines, but decreases their secretion in response to ATP treatment.
HSM extract suppresses ATP-induced caspase-1 activation in macrophages
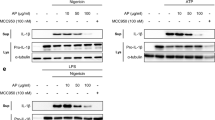
The cytokines IL-1β and IL-18 are generated as cytosolic precursors that require cleavage by the protease caspase-1 in order to generate biologically active cytokines. Caspase-1 itself is activated by several innate immune complexes termed inflammasomes37. To determine whether caspase-1 gene expression and activation are affected by the HSM ethanol extract, we pre-incubated THP-1 macrophages with HSM for 20 h prior to LPS and ATP treatments as mentioned above. As shown in Figure 4a and 4b , the HSM extract decreased caspase-1 mRNA expression in the activated macrophages treated with LPS and ATP. The HSM extract also significantly inhibited ATP-induced caspase-1 activation (secretion) in a dose-dependent manner ( Figure 4c and 4d ). These results suggest that the ability of HSM to decrease secretion of IL-1β and IL-18 is due at least in part to reduced caspase-1 gene expression and protein activation in the treated macrophages.
Effects of HSM on ATP-mediated caspase-1 gene expression and activation in human macrophages.
Cells were pretreated with HSM extracts (1 or 2%) for 20 h, followed by treatment with LPS (0.5 μg/ml) for 3 h and subsequently ATP (5 mM) for 1 h. (a) The mRNA expression levels of caspase-1 were determined by RT-PCR analysis. (b) Caspase-1 mRNAs were quantified using real-time PCR. β-actin gene expression was used for normalization. The results are expressed as fold changes, considering one as the value of untreated cells. (c) The secretion of caspase-1 subunit p20 into the supernatants of THP-1 macrophages was assessed by ELISA. (d) Cell lysates and culture supernatants were Western-blotted to detect pro-caspase-1 p45 and caspase-1 subunit p20. Data are presented as means ± SE of three experiments preformed in duplicate. #P < 0.01 versus untreated cells. *P < 0.01 versus HSM-untreated control (ethanol) cells. †P < 0.05 versus HSM (1%) treated cells.
HSM extract inhibits NLRP1 inflammasome expression and caspase-4 activation in ATP-treated macrophages
Several inflammasome complexes, including the NLRP1 and NLRP3 inflammasomes, have been shown to activate caspase-1. Canonical inflammasomes are formed by three components that include an NLR family protein (e.g., NLRP1 or NLRP3), the adaptor protein ASC and pro-caspase-158. To examine whether the NLRP1 and NLRP3 inflammasomes may be involved in the anti-inflammatory activity of HSM, we measured the mRNA and protein expression levels of NLRP1, NLRP3 and ASC using RT-PCR, quantitative real-time PCR and Western blot analyses. As shown in Figure 5a–c , pre-treatment of the cells with HSM decreased NLRP1 mRNA and protein levels compared to cells treated only with LPS and ATP. Conversely, the expression of NLRP3, which was clearly induced by LPS and ATP, was further increased in cells pretreated with HSM ( Figure 5a–c ). In addition, the level of ASC expression, which decreased following LPS and ATP treatments, appeared unchanged by HSM pre-treatment. These results suggest that the HSM extract may reduce IL-1β and IL-18 secretion by down-regulating NLRP1.
Effects of HSM on inflammasome components and caspase-4 activation in human macrophages.
Cells were pretreated with various concentrations (1 or 2%) of HSM extract for 20 h, followed by treatment with LPS (0.5 μg/ml) for 3 h and ATP (5 mM) for 1 h. (a) The mRNA expression levels of ASC, NLRP1, NLRP3 and caspase-4 were determined by RT-PCR, using β-actin as the internal control. (b) ASC, NLRP1, NLRP3 and caspase-4 mRNAs were quantified using real-time PCR. β-actin gene expression was used for normalization. The results are expressed as fold changes, considering one as the value of untreated cells. (c) Cell lysates were analyzed by Western blot analysis using specific anti-ASC, anti-NLRP1 and anti-NLRP3 antibodies. (d) Cell lysates were analyzed for protein levels of caspase-4 by Western blot analysis. β-actin was used as an internal control. Data are presented as means ± SE of three experiments preformed in duplicate. #P < 0.01 versus untreated cells.  versus HSM-untreated control (ethanol) cells. *P < 0.01 versus HSM-untreated control (ethanol) cells.
versus HSM-untreated control (ethanol) cells. *P < 0.01 versus HSM-untreated control (ethanol) cells.
Recent studies have shown that pro-IL-1β and pro-IL-18 are also substrates of non-canonical inflammasomes containing the protease caspase-4 and that this proteolytic enzyme is able to generate the biologically active form of the cytokines56,59. To determine whether the HSM extract may affect caspase-4 gene expression in macrophages treated with LPS and ATP, we performed RT-PCR and quantitative real-time PCR assays using caspase-4 specific primers. As shown in Figure 5a and 5b , pre-treatment with HSM resulted in a significant decrease of caspase-4 gene expression in LPS-primed macrophages stimulated with ATP. Western blot analysis of HSM-treated cells also showed a significant reduction of active caspase-4 in cell lysates ( Figure 5d ). Taken together, these results suggest that the HSM-mediated reduction of IL-1β and IL-18 secretion by macrophages is due to both down-regulation of the NLRP1 inflammasome and decreased activity of caspase-4.
HSM extract down-regulates P2X7 receptor expression and ROS production in LPS-primed and ATP-stimulated macrophages
Previous studies have shown that activation of the purinergic receptor P2X7R by extracellular ATP is required for caspase-1 activation, a process that leads to processing and release of both caspase-1 and mature IL-1β into the culture medium of activated macrophages60,61,62.
To determine whether the HSM extract may influence expression of P2X receptors (i.e., ATP-gated channels), we pre-treated THP-1 macrophages with HSM extract (1 or 2%) and then with LPS and ATP, prior to measuring the mRNA and protein expression levels of P2X7R and P2X4R (receptor for lower concentrations of ATP than P2X7R) using RT-PCR, quantitative real-time PCR and Western blot analyses. As shown in Figure 6a–c , the up-regulation of P2X7R expression induced by ATP (and LPS) was suppressed in a dose-dependent manner by pre-treatment by the HSM extract. In comparison, the up-regulation of P2X4R expression induced by ATP was not affected by HSM treatment.
Effects of HSM on the expression of P2X4R and P2X7R and ROS production in human macrophages.
Cells were pretreated with various concentrations (1 or 2%) of HSM extract for 20 h, followed by treatment with LPS (0.5 μg/ml) for 3 h and ATP (5 mM) for 1 h. (a) The mRNA expression levels of P2X4R and P2X7R were determined by RT-PCR, using β-actin as the internal control. (b) P2X4R and P2X7R mRNAs were quantified using real-time PCR. β-actin gene expression was used for normalization. The results are expressed as fold changes, considering one as the value of untreated cells. (c) Cell lysates were analyzed by Western blot analysis used specific anti-P2X4R and anti-P2X7R antibodies. (d) ROS production was measured with the total ROS detection kit, using a fluorescence microplate reader. Pyocyanin (200 μM), a ROS inducer, was used as a positive control for ROS formation. Data are presented as means ± SE of three experiments preformed in duplicate. #P < 0.01 versus untreated cells.  versus HSM-untreated control (ethanol) cells. *P < 0.01 versus HSM-untreated control (ethanol) cells. †P < 0.05 versus HSM (1%) treated cells.
versus HSM-untreated control (ethanol) cells. *P < 0.01 versus HSM-untreated control (ethanol) cells. †P < 0.05 versus HSM (1%) treated cells.
Previous studies have reported that activation of the P2X7R by ATP induces the production of ROS which are also required for activation of caspase-1 and secretion of IL-1β and IL-1863,64. To test whether the ROS production induced by ATP is also affected by HSM, we used a commercially-available detection kit to measure ROS production in macrophages that were pre-treated with HSM, prior to LPS priming and ATP activation. ATP treatment in LPS-primed macrophages caused a significant increase of ROS production compared with untreated cells ( Figure 6d ; pyocyanin was used as a positive control for ROS formation). The increase in ROS production could be suppressed by pre-treating macrophages with HSM extract ( Figure 6d ). Taken together, these results indicate that the HSM ethanol extract reduces IL-1β and IL-18 secretion in activated macrophages in part by down-regulating the ATP purinergic receptor P2X7R and reducing ATP-induced ROS production.
Discussion
C. sinensis is a well-known traditional Chinese medicinal mushroom used for the treatment of a variety of human diseases such as liver disease, respiratory disease, renal dysfunction, heart disease, hyperglycemia and hyperlipidaemia6,7. Recent studies have demonstrated that C. sinensis possesses immunomodulatory properties that, depending on the context, both activate and inhibit the immune system12,65,66. In the present study, we evaluated the effects of an HSM ethanol extract on the secretion of IL-1β and IL-18 induced by ATP in LPS-primed macrophages. We observed that pre-treatment with the HSM ethanol extract reduced the production of pro-inflammatory cytokines IL-1β and IL-18 in these cells. This finding is consistent with previous reports showing that C. sinensis extracts down-regulate the production of IL-1β in other LPS-activated immune cells24,25. However, our results also show that the HSM extract increases the transcription levels of the IL-1β and IL-18 precursors in LPS-primed and ATP-stimulated macrophages. Previous studies have reported that Cordyceps militaris, another Cordyceps species which is different from both natural C. sinensis and cultured HSM, induces IL-1β and IL-18 mRNA expression in murine RAW264.7 macrophages67,68. Our results indicate that the HSM extract suppresses the production of IL-1β and IL-18 through a mechanism other than inhibition of mRNA expression.
Since the inflammasomes are involved in IL-1β and IL-18 secretion, we investigated whether the reduced IL-1β and IL-18 secretion in HSM-treated cells was mediated by these molecular complexes. Assembly of the inflammasomes results in activation of the protease, caspase-1. Activated caspase-1 is responsible for processing of pro-IL-1β and pro-IL-18 and secretion of the mature cytokines37. Our results show that LPS-primed macrophages pre-incubated with the HSM extract causes decreased activation of caspase-1 in ATP-treated macrophages. Treatment with HSM also decreases caspase-1 mRNA expression.
Greten et al. demonstrated previously that activation of the nuclear transcription factor NF-κB induces pro-IL-1β mRNA synthesis and inhibits caspase-1 activation in macrophages69. In addition, NF-κB is activated in response to various inflammatory stimuli, including bacterial LPS, cytokines and viral infection70. Based on these results, we suggest that the HSM extract may activate NF-κB and lead to induction of pro-inflammatory cytokines IL-1β and IL-18 precursors and to inhibition of capase-1 activation in macrophages treated with LPS and ATP.
Our mechanistic studies show that HSM-dependent reduction of IL-1β and IL-18 production is due to a specific down-regulation of the NLRP1 inflammasome and subsequent inhibition of caspase-1 activity. A recent study by Hsu et al. showed that MDP stimulation induces the association of NOD2 with NLRP1 to form a complex that activates caspase-1 and triggers processing and secretion of IL-1β in macrophages71. NLRP1 also plays a crucial role in Bacillus anthracis-induced IL-1β secretion71. Additionally, THP-1 monocytes that were differentiated into macrophages with phorbol 12-myristate 13-acetate (PMA) and further treated with LPS or MDP plus ATP induced NLRP1 inflammasome assembly, caspase-1 activation and IL-1β secretion52,53.
To our knowledge, our study is the first report demonstrating the effects of HSM extract on an inflammasome in THP-1 macrophages activated with LPS and ATP. Furthermore, we make the unexpected observation that a mushroom used in traditional medicine can also activate a non-canonical inflammasome. However, the HSM ethanol extract studied here also increased NLRP3 mRNA and protein levels in the activated macrophages. Recent evidence indicates that NLRP3 expression is tightly controlled by the activation of NF-κB and that NF-κB inhibition leads to a dose-dependent reduction of NLRP3 protein induced by LPS72. This finding further supports the possibility that the HSM extract may induce NF-κB activation, which also increases NLRP3 expression and induces accumulation of IL-1β and IL-18 precursors in LPS-primed and ATP-stimulated macrophages.
ATP-induced P2X7R activation promotes the production of ROS, which in turn stimulates activation of the NLRP3 inflammasome63. In this study, we demonstrated that pre-treatment of LPS-primed macrophages with HSM extract significantly inhibits ATP-induced P2X7R expression. Moreover, our results show that ATP-induced ROS production is suppressed by the HSM extract. In agreement with these findings, previous studies have shown that the H. sinensis preparation CorImmune displays antioxidant activity and protects tissues and cells against free radical-induced damage73,74. Anti-oxidant activity was also reported for natural C. sinensis and this activity might be derived partly from the polysaccharide fraction of C. sinensis water extracts75. Recently, caspase-4 expression was shown to be required for caspase-1 activation and maturation of pro-IL-1β and pro-IL-18 in keratinocytes and activated THP-1 macrophages, suggesting that caspase-4 may act upstream of a non-canonical inflammasome56. Interestingly, production of ROS plays an important role in ER stress induction, which further leads to proteolytic cleavage of caspase-476,77. To address whether caspase-4 expression and activation is also regulated by HSM, we examined the expression of caspase-4 in activated macrophages. Pre-treatment of the cells with HSM resulted in a significant reduction of caspase-4 expression and activation compared with control, untreated cells. These results indicate that HSM compounds may act upstream of the inflammasome and result in down-regulation of caspase-1 activation and reduced IL-1β and IL-18 secretion.
We are currently investigating the compounds responsible for producing the anti-inflammatory effects of HSM. The nucleoside derivative 3′-deoxyadenosine—also called “cordycepin”—has been described in the past as an active ingredient of C. sinensis extracts78,79,80. Studies have shown that synthetic cordycepin produces anti-inflammatory effects on cultured cells81,82,83. However, chemical analyses performed by other groups showed that, while cordycepin is found in C. militaris, this compound is usually absent in both natural C. sinensis fruiting bodies and cultured HSM84,85. In fact, our own high-performance liquid chromatography analysis confirmed that cordycepin is not detected in the HSM ethanol extract studied here (Y.-F. Ko, J. D. Young, unpublished observations). Our preliminary chemical analysis of the HSM ethanol extract indicates that the compounds responsible for the anti-inflammatory effects of HSM have molecular weights ranging from 400 to 1,500 Da, but that neither polysaccharides nor adenosine can be detected in the extract (Y.-F. Ko, J. D. Young, unpublished observations). Structural studies are in progress in our laboratories to identify the chemical nature of this anti-inflammatory activity.
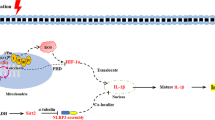
In conclusion, our results demonstrate that the HSM extract is a potent inhibitor of ATP-induced caspase-1 activation and secretion of IL-1β and IL-18 in LPS-primed human macrophages. Figure 7 summarizes the intracellular pathways affected by the HSM extract. The reduction of IL-1β and IL-18 secretion by the HSM ethanol extract in activated macrophages is associated with inhibition of P2X7R expression, ROS production, NLRP1 expression and caspase-1 and caspase-4 activation.
Schematic model for the reduction of IL-1β and IL-18 secretion in LPS-primed and ATP-stimulated macrophages treated with HSM.
The HSM extract down-regulated P2X7R expression, ROS production, NLRP1 expression and caspase-1 and caspase-4 activation, which together inhibited the secretion of IL-1β and IL-18. TLR4: Toll-like receptor 4.
The cytokines IL-1β and IL-18 can induce inflammation, fever and tissue damage in humans. Blocking secretion of these cytokines with HSM could represent a viable strategy to relieve symptoms associated with inflammatory disorders such as asthma, rheumatoid arthritis, inflammatory bowel disease and other autoimmune diseases.
Methods
Chemicals and reagents
ATP, LPS and PMA were purchased from Sigma-Aldrich (St. Louis, MO). Cell culture medium (RPMI 1640), FBS, penicillin and streptomycin were purchased from Life Technologies (Grand Island, NY). For Western blot analysis, the antibodies against IL-1β and caspase-4 were obtained from Cell Signaling Technology (Beverly, MA); the ones against ASC, P2X7R, pro-IL-1β and IL-18 were from Santa Cruz Biotechnology (Santa Cruz, CA); and those against NLRP3 and P2X4R were from Sigma-Aldrich. The antibody directed against caspase-1 was purchased from Millipore (Billerica, MA); the one against NLRP1 was from Enzo Life Sciences (Farmingdale, NY); and the one against β-actin was from Novus Biologicals (Littleton, CO). The secondary antibodies used were horseradish peroxidase-conjugated anti-rabbit and anti-mouse IgGs (Santa Cruz Biotechnology).
Fungal strain and preparation of the ethanol extract
The H. sinensis strain originally selected and characterized at Chang Gung Biotechnology (Taipei, Taiwan) was validated by comparison of its internal transcribed spacer DNA with that of natural C. sinensis23. The ethanol extract was prepared by adding 400 g of H. sinensis mycelium powder to 10 liters of 95% ethanol (v/v) into a Buchi R220 vacuum concentrator (Zurich, Switzerland), followed by stirring at a speed of 120 rpm for 60 min at 80°C. The HSM solution was cooled to room temperature and centrifuged at 4,500 rpm for 30 min at 4°C using a Sorvall RC 3C Plus centrifuge (Thermo Fisher Scientific, Waltham, MA). The supernatant was collected and concentrated to a final volume of 2 liters by using the Buchi R220 vacuum concentrator at 65°C. The HSM ethanol extract was finally sterilized by filtration through a 0.45 μm filter (Millipore) and stored at 4°C in dark glass bottles until use.
Cell culture and treatments
Human acute monocytic leukemia THP-1 cells (American Type Culture Collection, TIB-202) were cultured in RPMI 1640 medium supplemented with 10% (v/v) heat-inactivated FBS, 100 units/ml of penicillin and 100 μg/ml of streptomycin. THP-1 cells were incubated at 37°C in a cell culture incubator containing 5% CO2 and saturated humidity. The experiments were performed with cells plated in 6-well plates at 2 × 106 cells per well. The cells were differentiated to adherent macrophages by overnight culture in complete medium supplemented with 500 ng/ml of PMA and then with fresh complete medium for an additional 2 days. THP-1 macrophages were pre-treated for 20 h with 1 or 2% of HSM extract or with 2% ethanol as a control, followed by treatments with LPS (0.5 μg/ml) for 3 h and ATP (5 mM) for 1 h. Cell culture supernatants were harvested at 14,000 × g for 5 min at 4°C and the supernatants were collected and stored at –80°C for cytokine assay. In addition, cell lysates were resuspended in lysis buffer for RNA extraction and Western blot analysis.
MTT assay for cell viability
Cell viability was determined using a commercial MTT-based cytotoxicology test kit (Sigma-Aldrich), which detects viable cells colorimetrically based on the detection of the purple formazan compound produced by viable cells. THP-1 cells were initially seeded in 96-well plates (1 × 105 cells/well) for 24 h. For macrophage differentiation, cells were treated and incubated with PMA as described above. Cell culture media were replaced by complete media containing different concentrations of HSM extract ranging from 1 to 5%, followed by incubation for 24 h. After incubation, 10 μl of MTT (5 mg/ml) were added to each well and the plates were incubated at 37°C for 4 h. Each well was eluted and the precipitates were dissolved with 100 μl of MTT solubilization solution. Cell viability was obtained by calculating absorption values at 570 nm using a VersaMax microplate ELISA reader (Sunyvale, CA). All treated samples and controls were tested in triplicate.
Enzyme linked immuno sorbent assay (ELISA)
THP-1 macrophages (2 × 106 cells/well) in 6-well culture plates were pre-incubated with the HSM extract (1 or 2%) in 1 ml of complete medium for 20 h, followed by treatment with LPS (0.5 μg/ml) for 3 h and treatment with ATP (5 mM) for 1 h. Cell culture supernatants were collected and centrifuged at 10,000 × g, 4°C for 5 min to remove cell debris. Levels of secreted IL-1β, IL-18 and activated caspase-1 in cell culture supernatants were measured using commercially available ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
Measurement of ROS production
Total ROS/Superoxide detection kit (Enzo Life sciences) was used to assess ROS production in THP-1 macrophages. Briefly, cells were first seeded (1 × 105 cells/well) in 96-well culture plates for 24 h. For macrophage differentiation, cells were treated and incubated with PMA in as described above. Cell media were replaced by complete media containing different concentrations of HSM extract (1 or 2%) and then incubated for 20 h, followed by treatment with LPS (0.5 μg/ml) for 3 h and treatment with ATP (5 mM) for 1 h. In addition, cells were treated with the ROS inducer pyocyanin (200 μM), as a positive control, for 30 min at 37°C. After treatment, cells were washed with 200 μl of 1× wash buffer and loaded with 100 μl of ROS/Superoxide detection reagents and then incubated at 37°C for 1 h. The plates were read using a VersaMax microplate ELISA reader (Sunyvale, CA) at 520 nm after excitation at 488 nm. The increase in relative fluorescence intensity was used to determine intracellular ROS production.
Protein extraction and western blot analysis
Cell extracts and cell culture supernatants were analyzed by Western blot analysis. Twenty hours after HSM treatment, cells were treated with LPS (0.5 μg/ml) for 3 h and subsequently with ATP (5 mM) for 1 h. The HSM-treated cells were washed twice with PBS and suspended in RIPA lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.25% deoxycholic acid, 1% Nonidet P-40, 1 mM EDTA) (Millipore) and complete protease inhibitor cocktail (Roche, Mannheim, Germany). Cell suspensions were incubated on ice for 30 min and centrifuged at 15,000 × g for 30 min at 4°C. The supernatants of cell suspensions were harvested as described above and stored at –80°C. Total protein concentration was determined using the Bio-Rad Bradford assay (Herculus, CA). Proteins were separated by electrophoresis in 8-to-12% SDS-polyacrylamide gels and transferred onto Millipore PVDF membranes. Specific proteins were detected using the appropriate primary and secondary antibodies before visualization using enhanced chemiluminescence detection kit (Millipore).
RNA isolation and reverse transcriptase-polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from THP-1 cells using total RNA mini-kit according to the manufacturer's instructions (Geneaid, Taipei, Taiwan). Two μg of RNA were reversely transcribed in a reaction volume of 20 μl which contained an oligo (dT) primer, dNTP and the SuperScript™ III reverse transcriptase (Invitrogen, Carlsbad, CA). The cDNA for ASC, caspase-1, caspase-4, IL-1β, IL-18, NLRP1, NLRP3, R2X4R, P2X7R and β-actin were amplified by PCR using the following specific primers: ASC forward primer 5′-ATCCAGGCCCCTCCTCAGT-3′ and reverse primer 5′-GTTTGTGACCCTCCGCGATAAG-3′; caspase-1 forward primer 5′-GAATGTCAAGCTTTGCTCCCTAGA-3′ and reverse primer 5′-AAGACGTGTGCGGCTTGACT-3′; caspase-4 forward primer 5′-GGTCATCATTGTCCAGGC-3′ and reverse primer 5′-CCATTGTGCTGTCTCTCC-3′; IL-1β forward primer 5′-AAAAGCTTGGTGATGTCTGG-3′ and reverse primer 5′-TTTCAACACGCAGGACAGG-3′; IL-18 forward primer 5′-GCTGAACCAGTAGAAGACAATTG-3′ and reverse primer 5′-ATCTGATTCCAGGTTTTCATCATCT-3′; NLRP1 forward primer 5′-ACCTGATCCCAAGTGACTGC-3′ and reverse primer 5′-TCTTCTCCAGGGCTTCGATA-3′; NLRP3 forward primer 5′-CTTCTCTGATGAGGCCCAAG-3′ and reverse primer 5′-GCAGCAAACTGGAAAGGAAG-3′; P2X4R forward primer 5′-GGATGTGGCGGATTATGTGATAC-3′ and reverse primer 5′-AGTGGTCGCATCTGGAATCTC-3′; P2X7R forward primer 5′-TGTGCCTACAGGTGCTACGCC-3′ and reverse primer 5′-GCCCTTCACTCTTCGGAAACTC-3′; and β-actin forward primer 5′-GAGACCTTCAACACCCCAGCC-3′ and reverse primer 5′-GGATCTTCATGAGGTAGTCAG-3′. Amplified PCR products were electrophoresed in a 2% agarose gel and visualized by ethidium bromide (Sigma-Aldrich) staining using a standard image system.
Quantitative real-time PCR analysis
Quantitative real-time PCR was performed using LightCycler technology (Roche) with FastStart DNA MasterPLUS SYBR Green I (Roche) detection. Each LightCycler capillary was loaded with a total volume of 20 μl containing template cDNA, 250 nM sense and antisense primers and 4 μl of 5× SYBR Green Master Mix. In all assays, cDNA was amplified using a standard program (10 min denaturing step; 50 amplification cycles of 10 s at 95°C, 10 s at 55°C and 10 s at 72°C). Real-time PCR was performed with the same primer sequences stated above. Relative quantification of target gene expression was determined using a mathematical model described in the manufacturer's guidelines (Roche). Each PCR assay was performed in triplicate on two separate occasions for each experiment.
Statistical analysis
Triplicate data for each experiment were presented as mean ± SE. Mean comparisons between HSM-treated and control untreated cells were analyzed using Student's t-test. P values below 0.05 were considered statistically significant.
References
Lindequist, U., Niedermeyer, T. H. & Jülich, W. D. The pharmacological potential of mushrooms. Evid. Based Complement. Alternat. Med. 2, 285–299 (2005).
Wasser, S. P. Current findings, future trends and unsolved problems in studies of medicinal mushrooms. Appl. Microbiol. Biotechnol. 89, 1323–1332 (2011).
Kinjo, N. & Zhang, M. Morphological and phylogenetic studies on Cordyceps sinensis distributed in southwestern China. Mycoscience 42, 567–574 (2001).
Stone, R. Mycology. Last stand for the body snatcher of the Himalayas? Science 322, 1182 (2008).
Paterson, R. R. Cordyceps: a traditional Chinese medicine and another fungal therapeutic biofactory? Phytochemistry 69, 1469–1495 (2008).
Zhu, J. S., Halpern, G. M. & Jones, K. The scientific rediscovery of the ancient Chinese herbal medicine: Cordyceps sinensis: part I. J. Altern. Complement. Med. 4, 289–303 (1998).
Zhu, J. S., Halpern, G. M. & Jones, K. The scientific rediscovery of the ancient Chinese herbal medicine: Cordyceps sinensis: part II. J. Altern. Complement. Med. 4, 429–457 (1998).
Pegler, D. N., Yao, Y. J. & Li, Y. The Chinese ‘caterpillar fungus'. Mycologist 8, 3–5 (1994).
Dong, C. H. & Yao, Y. J. On the reliability of fungal materials used in studies on Ophiocordyceps sinensis. J. Ind. Microbiol. Biotechnol. 38, 1027–1035 (2011).
Buenz, E. J., Bauer, B. A., Osmundson, T. W. & Motley, T. J. The traditional Chinese medicine Cordyceps sinensis and its effects on apoptotic homeostasis. J. Ethnopharmacol. 96, 19–29 (2005).
Yalin, W., Ishurd, O., Cuirong, P. & Yuanjiang, P. Structure analysis and antitumor activity of (1→3)-beta-d-glucans (cordyglucans) from the mycelia of Cordyceps sinensis. Planta Med. 71, 381–384 (2005).
Wu, Y., Sun, H., Qin, F., Pan, Y. & Sun, C. Effect of various extracts and a polysaccharide from the edible mycelia of Cordyceps sinensis on cellular and humoral immune response against ovalbumin in mice. Phytother. Res. 20, 646–652 (2006).
Koh, J. H. et al. Activation of macrophages and the intestinal immune system by an orally administered decoction from cultured mycelia of Cordyceps sinensis. Biosci. Biotechnol. Biochem. 66, 407–411 (2002).
Shahed, A. R., Kim, S. I. & Shoskes, D. A. Down-regulation of apoptotic and inflammatory genes by Cordyceps sinensis extract in rat kidney following ischemia/reperfusion. Transplant. Proc. 33, 2986–2987 (2001).
Rao, Y. K., Fang, S. H. & Tzeng, Y. M. Evaluation of the anti-inflammatory and anti-proliferation tumoral cells activities of Antrodia camphorata, Cordyceps sinensis and Cinnamomum osmophloeum bark extract. J. Ethnopharmacol. 114, 78–85 (2007).
Yamaguchi, Y., Kagota, S., Nakamura, K., Shinozuka, K. & Kunitomo, M. Antioxidant activity of the extracts from fruiting bodies of cultured Cordyceps sinensis. Phytother. Res. 14, 647–649 (2000).
Tsai, C. H., Stern, A., Chiou, J. F., Chern, C. L. & Liu, T. Z. Rapid and specific detection of hydroxyl radical using an ultraweak chemiluminescence emitter: application to hydroxyl radical-scavenging ability of aqueous extracts of food constituents. J. Agric. Food. Chem. 49, 2137–2141 (2001).
Kuo, C. F. et al. Cordyceps sinensis, mycelium protects mice from group A streptococcal infection. J. Med. Microbiol. 54, 795–802 (2005).
Ji, D. B. et al. Antiaging effect of Cordyceps sinensis extract. Phytother. Res. 23, 116–122 (2009).
Chen, Y. Q., Wang, N., Qu, L., Li, T. & Zhang, W. Determination of the anamorph of Cordyceps sinensis inferred from the analysis of the ribosomal DNA internal transcribed spacers and 5.8S rDNA. Biochem. Syst. Ecol. 29, 597–607 (2001).
Hao, J. W. Corbrin (CorImmune) in treatment of drug-induced leukocytopenia in renal transplant patients. Chin. J. New Drugs 7, 292–298 (1998).
Xun, C. et al. Radiation mitigation effect of cultured mushroom fungus Hirsutella Sinensis (CorImmune) isolated from a Chinese/Tibetan herbal preparation–Cordyceps Sinensis. Int. J. Radiat. Biol. 84, 139–149 (2008).
Chen, J. L., Chen, Y. C., Yang, S. H., Ko, Y. F. & Chen, S. Y. Immunological alterations in lupus-prone autoimmune (NZB/NZW) F1 mice by mycelia Chinese medicinal fungus Cordyceps sinensis-induced redistributions of peripheral mononuclear T lymphocytes. Clin. Exp. Med. 9, 277–284 (2009).
Kuo, Y. C. et al. Shiao. Regulation of bronchoalveolar lavage fluids cell function by the immunomodulatory agents from Cordyceps sinensis. Life Sci. 68, 1067–1082 (2001).
Li, C. Y. et al. Two-sided effect of Cordyceps sinensis on dendritic cells in different physiological stages. J. Leukoc. Biol. 85, 987–995 (2009).
Dinarello, C. A. Interleukin-1β, interleukin-18 and the interleukin-1β converting enzyme. Ann. N. Y. Acad. Sci. 856, 1–11 (1998).
Gregory, J. L. et al. Macrophage migration inhibitory factor induces macrophage recruitment via CC chemokine ligand 2. J. Immunol. 177, 8072–8079 (2006).
Sims, J. E. & Smith, D. E. The IL-1 family: regulators of immunity. Nat. Rev. Immunol. 10, 89–102 (2010).
Dinarello, C. A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27, 519–550 (2009).
Davis, B. K., Wen, H. & Ting, J. P. The inflammasome NLRs in immunity, inflammation and associated diseases. Annu. Rev. Immunol. 29, 707–735 (2011).
Dinarello, C. A. & Wolff, S. M. The role of interleukin-1 in disease. N. Engl. J. Med. 328, 106–113 (1993).
Dinarello, C. A. Biologic basis of interleukin-1 in disease. Blood 87, 2095–2147 (1996).
Church, L. D., Cook, G. P. & McDermott, M. Primer: inflammasomes and interleukin 1 beta in inflammatory disorders. Nat. Clin. Pract. Rheumatol. 4, 34–42 (2008).
Nakanishi, K., Yoshimoto, T., Tsutsui, H. & Okamura, H. Interleukin-18 regulates both Th1 and Th2 responses. Annu. Rev. Immunol. 19, 423–474 (2001).
Arend, W. P., Palmer, G. & Gabay, C. IL-1, IL-18 and IL-33 families of cytokines. Immunol. Rev. 223, 20–38 (2008).
Bianchi, M. E. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 81, 1–5 (2007).
Martinon, F., Burns, K. & Tschopp, J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 10, 417–426 (2002).
Pétrilli, V., Dostert, C., Muruve, D. A. & Tschopp, J. The inflammasome: a danger sensing complex triggering innate immunity. Curr. Opin. Immunol. 19, 615–622 (2007).
Franchi, L., Eigenbrod, T., Muñoz-Planillo, R. & Nuñez, G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 10, 241–247 (2009).
Bours, M. J., Swennen, E. L., Di Virgilio, F., Cronstein, B. N. & Dagnelie, P. C. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol. Ther. 112, 358–404 (2006).
Saïd-Sadier, N. & Ojcius, D. M. Alarmins, inflammasomes and immunity. Biomed. J. 35, 437–449 (2012).
Di Virgilio, F. Liaisons dangereuses: P2X7 and the inflammasome. Trends Pharmacol. Sci. 28, 465–472 (2007).
Yu, H. B. & Finlay, B. B. The caspase-1 inflammasome: a pilot of innate immune response. Cell Host Microbe 4, 198–208 (2008).
Dostert, C. et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320, 674–677 (2008).
Duewell, P. et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 (2010).
Feldmeyer, L. et al. The inflammasome mediates UVB-induced activation and secretion of interleukin-1bata by keratinocytes. Curr. Biol. 17, 1140–1145 (2007).
Hise, A. G. et al. Fitzgerald. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe 5, 487–497 (2009).
Hornung, V. et al. Silica crystals and aluminum salts activate the NLRP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9, 847–856 (2008).
Mariathasan, S. et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232 (2006).
Martinon, F., Petrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NLRP3 inflammasome. Nature 440, 237–241 (2006).
Willingham, S. B. et al. Microbial pathogen-induced necrotic cell death mediated by the inflammasome component CIAS1/cryopyrin/NLRP3 and ASC. Cell Host Microbe 2, 147–159 (2007).
Bruey, J. M. et al. Bcl-2 and Bcl-XL regulate proinflammatory caspase-1 activation with NALP1. Cell 129, 45–56 (2007).
Faustin, B. et al. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol. Cell 25, 713–724 (2007).
Broz, P. et al. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature 490, 288–291 (2012).
Kayagaki, N. et al. Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121 (2011).
Sollberger, G., Strittmatter, G. E., Kistowska, M., French, L. E. & Beer, H. D. Caspase-4 is required for activation of inflammasomes. J. Immunol. 188, 1992–2000 (2012).
Akhter, A. et al. Caspase-11 promotes the fusion of phagosomes harboring pathogenic bacteria with lysosomes by modulating actin polymerization. Immunity 37, 35–47 (2012).
Schroder, K. & Tschopp, J. The inflammasomes. Cell 140, 821–832 (2010).
Gaggero, A. et al. A novel isoform of pro-interleukin-18 expressed in ovarian tumors is resistant to caspase-1 and -4 processing. Oncogene 23, 7552–7560 (2004).
Perregaux, D. & Gabel, C. A. Interleukin-1β maturation and release in response to ATP and nigericin: evidence that potassium depletion mediated by these agents is necessary and common feature of their activity. J. Biol. Chem. 269, 15195–15203 (1994).
Mehta, V. B., Hart, J. & Wewers, M. D. ATP stimulated release of IL-1β and IL-18 required priming by LPS and is independent of caspase-1 cleavage. J. Biol. Chem. 276, 3820–3826 (2001).
Ferrari, D. et al. The P2X7 receptor: a key player in IL-1 processing and release. J. Immunol. 176, 3877–3883 (2006).
Cruz, C. M. et al. ATP activates a reactive oxygen species-dependent oxidative stress responses and secretion of proinflammatory cytokines in macrophages. J. Biol. Chem. 282, 2871–2879 (2007).
Coutinho-Silva, R., Corrêa, G., Sater, A. A. & Ojcius, D. M. The P2X7 receptor and intracellular pathogens: a continuing struggle. Purinergic Signal. 5, 197–204 (2009).
Ng, T. B. & Wang, H. X. Pharmacological actions of Cordyceps, a prized folk medicine. J. Pharm. Pharmacol. 57, 1509–1519 (2005).
Zhou, X. et al. Cordycepin is an immunoregulatory active ingredient of Cordyceps sinensis. Am. J. Chin. Med. 36, 967–980 (2008).
Kim, C. S. et al. Cordyceps militaris induces the IL-18 expression via its promoter activation for IFN-gamma production. J. Ethnopharmacol. 120, 366–371 (2008).
Lee, J. S. et al. Study of macrophages activation and structural characteristics of purified polysaccharide from fruiting body of Cordyceps militaris. J. Microbiol. Biotechnol. 20, 1053–1060 (2010).
Greten, F. R. et al. NF-κB is a negative regulator of IL-1β secretion as revealed by genetic and pharmacological inhibition of IKKβ. Cell 130, 918–931 (2007).
Yamamoto, Y. & Gaynor, R. B. Therapeutic potential of inhibition of the NF-κB pathway in the treatment ofinflammation and cancer. J. Clin. Invest. 107, 135–142 (2001).
Hsu, L. C. et al. A NOD2-NALP1 complex mediates caspase-1-dependent IL-1β secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc. Natl. Acad. Sciences. U. S. A. 105, 7803–7808 (2008).
Bauernfeind, F. G. et al. Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183, 787–791 (2009).
Yang, Z. H. & Ling, Y. S. Anti-oxidant and free radical effect of Corbrin (CorImmune). Guangdong J. Pharmacol. 13, 35–37 (1997).
Chen, S. J., Zhang, Z. H., An, L. H., Dong, G. F. & Yong, A. N. The change of free radical in the serum of patients with viral hepatitis and Corbrin (CorImmune) capsule's effect on the free radicals. Shangdong Med. Pharmaceut. J. 40, 15–19 (2000).
Li, S. P., Li, P., Dong, T. T. & Tsim, K. W. Anti-oxidation activity of different types of natural Cordyceps sinensis and cultured Cordyceps mycelia. Phytomedicine 8, 207–212 (2001).
Yokouchi, M. et al. Involvement of selective reactive oxygen species upstream of proapoptotic branches of unfolded protein response. J. Biol. Chem. 283, 4252–4260 (2008).
Hitomi, J. et al. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Abeta-induced cell death. J. Cell Biol. 165, 347–356 (2004).
Lui, J. C. et al. Cordycepin induced eryptosis in mouse erythrocytes through a Ca2+-dependent pathway without caspase-3 activation. Arch. Toxicol. 81, 859–865 (2007).
Leu, S. F., Poon, S. L., Pao, H. Y. & Huang, B. M. The in vivo and in vitro stimulatory effects of cordycepin on mouse leydig cell steroidogenesis. Biosci. Biotechnol. Biochem. 75, 723–731 (2011).
Li, L., He, D., Yang, J. & Wang, X. Cordycepin inhibits renal interstitial myofibroblast activation probably by inducing hepatocyte growth factor expression. J. Pharmacol. Sci. 117, 286–294 (2011).
Kim, H. G. et al. Cordycepin inhibits lipopolysaccharide-induced inflammation by the suppression of NF-kappaB through Akt and p38 inhibition in RAW 264.7 macrophage cells. Eur. J. Pharmacol. 545, 192–199 (2006).
Noh, E. M. et al. Cordycepin inhibits IL-1beta-induced MMP-1 and MMP-3 expression in rheumatoid arthritis synovial fibroblasts. Rheumatology (Oxford). 48, 45–48 (2009).
Jeong, J. W. et al. Anti-inflammatory effects of cordycepin via suppression of inflammatory mediators in BV2 microglial cells. Int. Immunopharmacol. 10, 1580–1586 (2010).
Li, S. P., Yang, F. Q. & Tsim, K. W. Quality control of Cordyceps sinensis, a valued traditional Chinese medicine. J. Pharm. Biomed. Anal. 41, 1571–1584 (2006).
Yang, F. Q. & Li, S. P. Effects of sample preparation methods on the quantification of nucleosides in natural and cultured Cordyceps. J. Pharm. Biomed. Anal. 48, 231–235 (2008).
Acknowledgements
This work was supported by Grant NSC-101-2321-B-002-009 from National Science Council and Grant CMRPD190303 from Chang Gung Memorial Hospital, Taiwan.
Author information
Authors and Affiliations
Contributions
T.-T.H., K.-Y.C., D.M.O., H.-C.L. and J.D.Y. conceived and designed the research. T.-T.H., Y.-H.W. and C.-Y.W. performed experiments. T.-T.H., Y.-H.W., Y.-F.K., C.-Y.W., J.M., C.-C.L. and H.-C.L. analyzed the data. T.-T.H., K.-Y.C., D.M.O., J.M., H.-C.L. and J.D.Y. wrote the manuscript.
Ethics declarations
Competing interests
Y.-F.K. is President and employee of Chang Gung Biotechnology Corporation. J.D.Y. is Chairman of the Board of Chang Gung Biotechnology Corporation. The other authors declare that no potential conflict of interest exists.
Rights and permissions
This work is licensed under a Creative Commons Attribution 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by/3.0/
About this article
Cite this article
Huang, TT., Chong, KY., Ojcius, D. et al. Hirsutella sinensis mycelium suppresses interleukin-1β and interleukin-18 secretion by inhibiting both canonical and non-canonical inflammasomes. Sci Rep 3, 1374 (2013). https://doi.org/10.1038/srep01374
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep01374
This article is cited by
-
Anti-inflammatory Effects of Novel P2X4 Receptor Antagonists, NC-2600 and NP-1815-PX, in a Murine Model of Colitis
Inflammation (2022)
-
H. sinensis mycelium inhibits epithelial-mesenchymal transition by inactivating the midkine pathway in pulmonary fibrosis
Frontiers of Medicine (2021)
-
Inhibition of the NLRP3-inflammasome prevents cognitive deficits in experimental autoimmune encephalomyelitis mice via the alteration of astrocyte phenotype
Cell Death & Disease (2020)
-
Hirsutella sinensis inhibits NLRP3 inflammasome activation to block aristolochic acid-induced renal tubular epithelial cell transdifferentiation
Human Cell (2020)
-
Knockdown of c-MET induced apoptosis in ABCB1-overexpressed multidrug-resistance cancer cell lines
Cancer Gene Therapy (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.