Key Points
-
Investigates the efficacy and tolerability of alcohol-free and alcohol-containing chlorhexidine mouthrinses.
-
Discusses the pharmacokinetics of alcohol-free and alcohol-containing chlorhexidine mouthrinses after single and repeated use.
Abstract
Objectives Gingival bleeding following twice-daily use of 0.2% w/v chlorhexidine digluconate mouthrinse with and without alcohol (0.2% CHX-alcohol; 0.2% CHX-alcohol-free, respectively) and brushing with a standard fluoride toothpaste was compared to brushing alone.
Methods Three hundred and nineteen subjects with mild-to-moderate gingivitis (with ≥16 gradable permanent teeth including four molars, bleeding after brushing and ≥20 bleeding sites) completed this randomised, examiner-blinded, parallel-group study. A prophylaxis was performed at baseline. Gingival Severity Index (GSI; primary objective), Gingival Index (GI) and Plaque Index (PI) were assessed at baseline and after 6 weeks of treatment. Adverse events (AEs) were recorded throughout the study.
Results Between treatment differences at week 6 demonstrated significantly lower GSI for the 0.2% CHX-alcohol and 0.2% CHX-alcohol-free groups compared to brushing alone (primary endpoint; treatment difference −0.061 [95% CI −0.081, −0.041] and −0.070 [95% CI −0.090, −0.050], respectively; both p <0.0001). There were also significant reductions in GI and PI for the 0.2% CHX-alcohol and 0.2% CHX-alcohol-free groups compared to brushing alone (all p <0.0001). The proportion of subjects reporting ≥1 treatment-related adverse events (TRAEs) was 27.8% (0.2% CHX-alcohol), 24.8% (0.2% CHX-alcohol-free) and 3.7% (brushing alone).
Conclusions Chlorhexidine mouthrinse with or without alcohol as an adjunct to brushing with regular fluoride toothpaste significantly reduces bleeding scores, plaque and gingival inflammation compared to brushing alone. TRAEs are characteristic of those associated with the use of chlorhexidine and are similar for both mouthrinses.
Similar content being viewed by others
Introduction
Dental plaque is a soft, sticky biofilm that forms naturally on the teeth and begins forming on clean tooth surfaces just a few minutes after brushing. If not removed, plaque can cause dental caries and periodontal diseases such as gingivitis and periodontitis.1,2,3
Mechanical methods such as tooth brushing with toothpaste and tooth flossing are the most reliable methods to remove plaque from the surfaces of teeth.4 Chlorhexidine mouthrinse, in combination with mechanical methods, has been demonstrated to reduce plaque and gingivitis scores.5
Objectives
The primary objective of this study was to compare gingival bleeding (Gingival Severity Index, GSI) following twice daily use of either chlorhexidine digluconate 0.2% w/v alcohol-containing mouthrinse (CHX-alcohol) plus brushing with standard fluoride toothpaste or chlorhexidine digluconate 0.2% w/v alcohol-free mouthrinse (CHX-alcohol-free) plus brushing with a standard fluoride toothpaste to brushing with a standard fluoride toothpaste (brushing alone) for 6 weeks. Secondary objectives were to compare the Gingival Index (GI) and Plaque Index (PI) following twice daily use of 0.2% CHX-alcohol mouthrinse or 0.2% CHX-alcohol-free mouthrinse and brushing with standard fluoride toothpaste compared to brushing alone for 6 weeks.
Adverse events and serious adverse events arising during the course of the study were recorded.
Materials and methods
Study design
The study was an examiner-blinded, randomised, parallel group design conducted at two sites in North West England. Prior to initiation the study was reviewed and approved by an independent ethics committee. The study protocol was registered at Clinicaltrials.gov (NCT01751178) and at http://www.gsk-clinicalstudyregister.com/study/RH01561#ps.
Healthy subjects aged 18 to 64 years were recruited. Subjects were recruited from the sites' databases and via advertisements. At the screening visit, written informed consent was obtained and subjects were provided with a standard fluoride toothpaste (Aquafresh (R) Mild & Minty, GSKCH, Weybridge, UK) and toothbrush (Aquafresh (R) Clean Control, GSKCH) and asked to brush as they normally would for one timed minute and to expectorate into a white cup. Evidence of blood in the expectorant or bleeding while brushing were the initial inclusion criteria. Additional inclusion/exclusion criteria used were: ≥16 permanent, gradable teeth, including four molars (protocol amendment 3: changed from 20 gradable teeth); and ≥20 or more bleeding sites at the baseline examination. Women who were pregnant, lactating or those of child bearing potential not practising a reliable method of contraception were excluded from the study. The use of systemic medications which would have an effect on gingival conditions within 14 days of gingival examinations was prohibited. Subjects who were taking antibiotics within two weeks before the screening visit or throughout the study were excluded, as were those on concomitant medication that, in the opinion of the investigator, might interfere with the outcome of the study. Other than having mild to moderate gingivitis, subjects were to be in good oral health with no active caries, no heavy calculus deposits and no more than five periodontal pockets measuring ≥5 mm in depth (protocol amendment 3: changed from more than 3 pockets >/- 5 mm in depth).
Eligible subjects were instructed to continue home use of the toothpaste and toothbrush provided and reappointed for a baseline visit no more than 7 days later. Subjects were instructed to abstain from brushing for a 12-hour period before the baseline visit. Baseline assessments included oral soft tissue (OST), Löe and Silness6 Gingival Index (GI) and the Turesky7 Modified Quigley Hein8 Plaque Index (PI). A complete dental prophylaxis, which included flossing to ensure removal of all plaque, was performed by an appropriately trained professional at the baseline visit or on a separate visit within 7 days of baseline. Saliva samples were collected from a subset of subjects (identified as the first 60 that were available for 4 hours after treatment) before prophylaxis and then at 30 min, 1, 2 and 4 hours post treatment.
Subjects were then stratified, according to the study site (Manchester or Wirral, UK), baseline number of bleeding sites and smoking status, and randomised into one of three treatment groups: CHX-alcohol plus standard fluoride toothpaste; CHX-alcohol-free plus standard fluoride toothpaste or brushing alone. Subjects (under supervision for first use) brushed their teeth with a full brush head of toothpaste for one timed minute, rinsed their mouth with water then waited for five timed minutes. After five minutes, they swished with 10 ml of their assigned mouthwash (mouthwash groups only) for a timed one minute followed by expectorating. All treatments were similarly applied twice daily for the next 6 weeks at home. For assessment of compliance, each home use of study treatments was recorded in the subjects' diary card. Subjects in the saliva subset returned to clinic one or two days before their scheduled 6 week visit to have samples taken pre-and post-dosing as per the baseline visit. All subjects returned to the study site (week 6) with overnight plaque accumulation for OST, GI and PI. All assessments of GI were performed by a single, experienced examiner.
Safety was analysed in terms of adverse events. Adverse events were coded using the current version of MedDRA. Treatment emergent adverse events (TEAEs) and treatment related adverse events (TRAEs) were tabulated and reviewed.
There were four protocol amendments during the study. The first amendment was to clarify inconsistencies within the protocol documentation and to remove the step that subjects in the saliva subset would not have their teeth disclosed during the dental prophylaxis in case this interfered with chlorhexidine determination in the saliva samples. The second amendment was to increase the number of subjects to be screened from approximately 550 to 755 healthy subjects. The third amendment was to revise the inclusion and exclusion criteria (see above). The fourth amendment allowed for up to 120 subjects randomised per group (rather than 120 subjects randomised).
Assessments
Saliva samples
Saliva samples (2 ml) were immediately frozen at −20 °C and transported to an external laboratory for determination of chlorhexidine levels by liquid chromatography mass spectrometry.
Oral soft tissue (OST)
An examination was made of the oral soft tissue including the labial mucosa, gingival mucosa, hard and soft palates, mucogingival folds, tongue, sublingual and submandibular areas, salivary glands, tonsilar and pharyngeal areas. Abnormal findings were recorded and included in the tabulation of adverse events.
Gingival Index (GI)
The gingival tissue was assessed by sweeping a blunt ended probe along the gingival margins and scored according to the GI6 at six sites on each tooth (distal, body and mesial for the buccal and lingual surfaces). The GI was scored as 0 (no inflammation), 1 (mild inflammation – slight change in colour, texture and no bleeding on probing), 2 (moderate inflammation – glazing, redness, ooedema and hypertrophy, bleeding on probing), 3 (severe inflammation – marked redness and hypertrophy, tendency for spontaneous bleeding). GI was calculated by taking the average over all measured tooth sites for a subject.
Gingival Severity Index (GSI)
The GSI (based on GI data with no separate clinical assessment) is a proportion reflecting the percentage of a subject's gingiva that is bleeding (rating from 0 to 1). According to the grading criteria, if the GI was scored 0 or 1 then the GSI was scored 0 (no bleeding). A GI score of 2 or 3 was scored as GSI 1 (bleeding). Therefore, an overall GSI score of 0 indicates that 0% of gingiva received a GI score ≥2. A score of 1 indicates that 100% of the gingiva had moderate to high levels of inflammation (bleeding). A GSI score of ≥0.5 indicates a considerable level of gingivitis.
Plaque Index (PI)
Plaque was disclosed and scored using the PI7,8 according to Soparkar's modification.9 Except for third molars, each tooth was divided into six areas including the mesiobuccal, buccal, distobuccal, mesiolingual, lingual and distolingual surfaces. The PI was scored as 0 (no plaque), 1 (slight flecks of plaque at the cervical margin of the tooth), 2 (a thin continuous band of plaque [1 mm or smaller] at the cervical margin of the tooth), 3 (a band of plaque wider than 1 mm but covering less than one third of the crown of the tooth), 4 (plaque covering at least one third but less than two thirds of the crown of the tooth), 5 (plaque covering two thirds or more of the crown of the tooth).
In order to assess reliability, the examiner completed one repeat plaque assessment on two subjects each day that plaque assessments were made. A repeat assessment was made after a minimum of 10 minutes had elapsed.
Statistical methods
The efficacy analysis was performed in the 'intent to treat' (ITT) population defined as randomised subjects who received study treatment and completed at least one post-baseline efficacy assessments. The 'per protocol' (PP) population was defined as those subjects in the ITT population who did not have any major protocol violations.
All statistical methods were carried out according to a null hypothesis of no difference between treatments. The comparisons of interest were 0.2% CHX-alcohol mouthrinse vs brushing alone and 0.2% CHX-alcohol-free mouthrinse vs brushing alone.
GSI at 6 weeks was the primary efficacy variable. Other variables of interest were GI, PI and interproximal PI at week 6. Kinetic variables included CHX levels in saliva at each time point, AUC0-4hrs (area under the curve), AUC0.5-4hrs, A0 (initial retention concentration) and Kel (elimination rate constant).
The GSI, GI and PI were calculated by taking the average over all measured tooth sites for a subject. The interproximal plaque score was calculated in the same way but was based solely on mesiobuccal, distobuccal, mesiolingual and distolingual surfaces.
All variables, except kinetic, were analysed using analysis of covariance (ANCOVA). The ANCOVA model included treatment group, site, smoking status (yes/no), strata level of number of bleeding sites and the appropriate baseline (GSI, GI, overall plaque or interproximal plaque) as a covariate. Treatment differences and 95% confidence intervals (95% CI) were presented. All tests were two sided and performed at the 5% significance level.
In order to assess repeatability of the examiner, a weighted kappa coefficient was calculated. Repeatability was deemed excellent if kappa was >0.75, fair to good if it was ≥0.4 or ≤0.75, and poor if it was <0.4. The kinetic variables were summarised by treatment group.
Safety
All subjects who were randomised and administered the study treatment were considered evaluable for the safety population. No statistical comparisons with respect to adverse events were performed.
Results
Disposition/demography
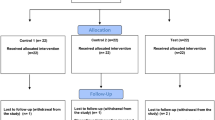
Seven hundred and seventy-five participants were screened with 324 randomised and 319 completing the study (ITT population, Fig. 1). The high screen failure rate was due mainly to insufficient numbers of gradeable teeth and pockets >5 mm, which was revised in protocol amendment 3. Of the five randomised subjects who discontinued, three were in the 0.2% CHX-alcohol group (two withdrew due to adverse events: joint dislocation [subject 1]; dysgeusia and paraesthesia oral [subject 2] and one withdrew consent); and two subjects withdrew consent in the brushing alone group. Most subjects were female (239 of 324; 73.8%) and White (280 of 324, 86.4%) with a mean age of 36.8 years (Table 1). Since there was >10% difference in the number of subjects in the ITT and PP populations a separate PP analysis was performed for the primary variable.
Efficacy
The effects of chlorhexidine with and without alcohol and brushing alone on GSI, GI and PI are presented in Figures 2a, 2b and 2c, respectively and in Table 2a. All efficacy variables were significantly lower for both chlorhexidine groups compared to the brushing alone group (treatment differences [95% CI]: GSI, −0.061 [-0.081, −0.041], −0.070 [-0.090, −0.050], respectively; GI, −0.08 [-0.10, −0.05], −0.08 [-0.11, −0.06], respectively; TPI, −0.80 [-0.98, −0.62], −0.86 [-1.04, −0.68], respectively; all p <0.0001; Table 2b). The repeatability analysis of the PI (based on 77 subjects) showed excellent agreement between the first and second scoring; the kappa value for PI was 0.891 (95% CI = 0.884,–0.889).
Analysis of GSI at 6 weeks for the PP population also showed scores for both chlorhexidine mouthrinse groups to be significantly lower compared to the brushing alone group (treatment difference [95% CI]; GSI, −0.050 [-0.071, −0.030], −0.063 [-0.084, −0.043] CHX-alcohol; CHX alcohol-free respectively; all p <0.0001).
Pharmacokinetics
Salivary levels of chlorhexidine and kinetic variables after a single and repeat dose of chlorhexidine mouthrinse are summarised in Table 3. As expected, levels are higher after nearly 6 weeks of repeated dosing. AUCs between the 0.2% CHX-alcohol and 0.2% CHX-alcohol-free mouthrinses were similar. After a single dose, the elimination rate and initial retention were similar between the two formulas; however, after repeat dosing, the initial retention was higher for the 0.2% CHX-alcohol group (84,010.90 vs. 68,208.62 ng/ml), but the elimination rate was slower for the 0.2% CHX-alcohol-free group (−0.15 vs. −0.08 1/hr).
Safety
A total of 338 TEAEs were recorded among 174 (53.7%) of subjects (Table 4a). A large number of TRAEs were recorded in the two chlorhexidine groups where 27.8% of subjects in the 0.2% CHX-alcohol group and 24.8% in the 0.2% CHX-alcohol-free group reported at least one TRAE compared to only 3.7% in the brushing alone group. The most commonly reported TRAEs were coated tongue, glossodynia, oral paraesthesia, ageusia, dry mouth, oral hypoaesthesia and dysgeusia (Table 4b).
There was one serious adverse event, not related to study product and one severe event (oral paresthesia) in the 0.2% CHX-alcohol group which did resolve. The subject withdrew from the study due to the event. A second subject withdrew from the study due to a non-TRAE.
Discussion
The results of this study demonstrated both CHX-alcohol-containing and CHX-alcohol-free mouthrinse groups to have significantly reduced GSI, GI and PI compared to brushing alone, after a dental prophylaxis and 6 weeks of treatment. These results are in agreement with the findings of a recent systematic evaluation of comparative clinical studies performed in a 25-year period which found chlorhexidine mouthrinses to be effective in controlling plaque and gingivitis and consistently effective as adjuncts to mechanical oral hygiene procedures.5
As one of the most thoroughly investigated chemical agents for the control of plaque and gingivitis, chlorhexidine has long been recognised as the gold standard among antimicrobial agents.10 Its antibacterial mode of action is thought to be due to rapid attraction of the positively charged chlorhexidine molecule to the negatively charged bacterial cell membrane leading to damage and leakage of intracellular components.11
In addition to bacteria, chlorhexidine also binds to surfaces within the mouth such as teeth, pellicle, tongue, oral mucosa and salivary proteins and is then slowly released into the oral fluid. This creates an environment where the antibacterial activity of chlorhexidine is maintained for up to several hours depending on factors such as dose, rinsing time, temperature, presence of teeth or prostheses, organic material and saliva pH.12,13 In the present study, the retention and elimination of chlorhexidine from saliva over a 4-hour time period were similar after an initial rinse with 10 ml of either the CHX-alcohol or CHX-alcohol-free preparation. After 6 weeks of repeated rinsing, chlorhexidine levels in saliva were higher in both groups, the CHX-alcohol group had higher initial peak chlorhexidine levels in saliva after rinsing, and chlorhexidine was eliminated from saliva more slowly over a 4-hour time period in the CHX-alcohol-free group. Despite this slight difference in kinetic profiles after repeated rinsing, the amount of chlorhexidine retained in the oral cavity after rinsing with 10 ml, as measured by AUC, was similar between the rinse containing alcohol and the alcohol-free rinse. It has been suggested that the dose of chlorhexidine is of considerable relevance to its efficacy.5 Results of the present study confirm that levels of chlorhexidine are retained in the oral cavity and the efficacy outcomes confirm rinsing with 10 ml of either preparation to be more effective for controlling plaque and gingivitis compared to brushing alone.
Chlorhexidine delivered in a mouthwash formulation with and without alcohol has long been known to exert useful effects against plaque and gingivitis.14,15 In a randomised, double blind study a 0.12% chlorhexidine/fluoride rinse as an adjunct to normal toothbrushing resulted in significant improvements of plaque and gingivitis scores versus the control rinse after 6 weeks.16 In another large-scale clinical trial, chlorhexidine mouthrinse exerted significant reductions in measures of plaque and gingivitis compared with thymol-containing mouthrinse after 14 days of treatment.17 Moreover, a randomised, double blind study conducted in general dental practices in the UK revealed that chlorhexidine mouthrinse significantly reduced plaque accumulation by 28% and gingival inflammation by 25% over a 12-week period.18 More recently, a chlorhexidine oral rinse, unlike the herbal rinse tested, significantly reduced mean GI, PI, and bleeding on probing at 1, 2, and 3 months of treatment versus the placebo.19 The results of the present study confirm that use of chlorhexidine mouthwash as supplement to fluoride toothpaste may successfully reduce plaque and improve gingivitis compared with brushing with fluoride toothpaste alone.
A high proportion of subjects in the chlorhexidine mouthrinse groups reported TRAEs characteristic of those known to be associated with prolonged chlorhexidine use including discoloration of the tongue and oral tissues, alteration of taste sensation, burning sensation of the mouth, dry mouth, oral desquamation and loss of sensitivity to sensory stimuli in the mouth. For instance, Charles et al.20 observed that chlorhexidine mouthrinse and essential oil mouthrinse demonstrated significant reductions of plaque and gingivitis versus the control, although chlorhexidine group subjects exhibited significantly greater extrinsic mouth stain by the end of the 6-month study, a finding commonly observed in other long-term studies.15,16 Available evidence suggests variation in the degree of staining from person to person and that it is caused by the precipitation of negatively charged dietary chromogens, such as those from coffee, tea or red wine, onto positively charged chlorhexidine adsorbed onto pellicle coated surfaces.21 When staining occurs on tooth surfaces it is extrinsic and removable by regular prophylaxis. Alteration of taste sensation, burning sensation of the mouth, dry mouth, oral desquamation and loss of sensitivity to sensory stimuli in the mouth have also been reported in the literature and generally resolve once use of chlorhexidine is discontinued.22
Conclusion
The twice daily use of chlorhexidine mouthrinse containing 0.2% w/v chlorhexidine digluconate with or without alcohol and brushing with standard fluoride toothpaste reduced plaque and gingival inflammation as measured by GSI, GI and PI after 6 weeks of treatment following a complete prophylaxis. Adverse events in the mouthrinse groups were characteristic of those known associated with prolonged chlorhexidine use.
Contributors
Anto Jose, Andrew Butler and Mary Lynn Bosma contributed to the design, conduct and reporting of the study. Robert Maclure, Patricia Rimmer and David Payne were involved in the acquisition of data for the study and protocol advice. All authors had access to the final study report, made contributions to the development of the manuscript, had final responsibility for the decision to submit and approved the version submitted.
Disclosure statement
Anto Jose, Andrew Butler, Mary Lynn Bosma are employees of GSK Consumer Healthcare. David Payne, Robert Maclure and Patricia Rimmer are employees of Intertek Clinical Research Services, Wirral and Manchester, UK which has received funding from GSK Consumer Healthcare.
References
Loë H, Theidale E, Jensen S B . Experimental gingivitis in man. J Peridontol 1965; 36: 177–187.
Theilade E, Wright W H, Jensen S B, Loë H . Experimental gingivitis in man II. A longitudinal clinical and bacteriological investigation. J Periodontal Res 1966; 1: 1–13.
Timmerman M F, Van der Weijen G A . Risk factors for periodontitis. Int J Dent Hyg 2006; 4: 2–7.
Van der Weijen F, Slot D E . Oral hygiene in the prevention of periodontal diseases: the evidence. Periodontol 2000 2011; 55: 104–123.
Van strydonck D A C, Slot D E, Van der Velden U, Van der Weijen F . Effect of a chlorhexidine mouthrinse on plaque, gingival inflammation and staining in gingivitis patients: a systematic review. J Clin Periodontol 2012; 39: 1042–1055.
Löe H, Silness J . Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odont Scand 1963; 21: 533–551.
Turesky S, Gilmore N D, Glickman I . Reduced plaque formation by the chloromethyl analogue of victamine C. J Periodontol 1970; 41: 41–44.
Quigley G A, Hein J W . Comparative cleansing efficiency of manual power brushing. J Am Dent Assoc 1962; 65: 26–29.
Lobene R R, Soparkar P M, Newman M B . Use of dental floss. Effect on plaque and gingivitis. Clin Prev Dent 1982; 4: 5–8.
Jones C G . Chlorhexidine; is it still the gold standard? Periodontol 2000 1997; 15: 55–62.
Denton G W . Chlorhexidine. In Block S S (ed) Disinfection, sterilization and preservation. 4th ed. pp 274–289. Philadelphia: Lea and Febiger, 1991.
Roberts W R, Addy M . Comparison of the bisbiguanide antiseptics alexidine and chlorhexidine. I. Effect on plaque accumulation and salivary bacteria. J Clin Periodontol 1981; 8: 213–219.
Tomas I, Cousido M C, Garcia-Caballero L, Rubido S, Limeres J, Diz P . Substantivity of a single chlorhexidine mouthwash on salivary flora: influence of intrinsic and extrinsic factors. J Dent 2010; 38: 541–546.
Leyes Borrajo J L, Garcia V L, Lopez C G, Rodriguez-Nuñez I, Garcia F M, Gallas T M . Efficacy of chlorhexidine mouthrinses with and without alcohol: a clinical study. J Periodontol 2002; 73: 317–321.
Lorenz K, Bruhn G, Heumann C, Netuschil L, Brecx M, Hoffmann T . effect of two new chlorhexidine mouthrinses on the development of dental plaque, gingivitis, and discolouration. A randomised, investigator-blind, placebo-controlled, 3week experimental gingivitis study. J Clin Periodontol 2006; 33: 561–567.
Jenkins S, Addy M, Newcombe R . Evaluation of a mouthrinse containing chlorhexidine and fluoride as an adjunct to oral hygiene. J Clin Periodontol 1993; 20: 20–25.
Maruniak J, Clark W B, Walker C B et al. The effect of 3 mouthrinses on plaque and gingivitis development. J Clin Periodontol 1992; 19: 19–23.
Eaton K A, Rimini F M, Zak E et al. The effects of a 0.12% chlorhexidinedigluconatecontaining mouthrinse versus a placebo on plaque and gingival inflammation over a 3month period. A multicentre study carried out in general dental practices. J Clin Periodontol 1997; 24: 189–197.
Southern E N, McCombs G B, Tolle S L, Marinak K . The comparative effects of 0.12% chlorhexidine and herbal oral rinse on dental plaque-induced gingivitis. J Dent Hyg 2006; 80: 12.
Charles C H, Mostler K M, Bartels L L, Mankodi S M . comparative antiplaque and antigingivitis effectiveness of a chlorhexidine and an essential oil mouthrinse: 6month clinical trial. J Clin Periodontol 2004; 31: 878–884.
Watts A, Addy M . Tooth discolouration and staining: a review of the literature. Br Dent J 2001; 190: 309–316.
Varoni E, Tarce M, Lodi G, Carrassi A . Chlorhexidine in dentistry: state of the art. Minerva Stomatol 2012; 61: 399–419.
Acknowledgements
The authors thank Claire Lipscombe (Intertek) for her project management of this study, John Ward (GSK) for his overall study management and Linda Gendreau for assistance with writing this manuscript. This study and writing support for the manuscript were funded by GSK Consumer Healthcare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Refereed Paper
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Jose, A., Butler, A., Payne, D. et al. A randomised clinical study to evaluate the efficacy of alcohol-free or alcohol-containing mouthrinses with chlorhexidine on gingival bleeding. Br Dent J 219, 125–130 (2015). https://doi.org/10.1038/sj.bdj.2015.592
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bdj.2015.592