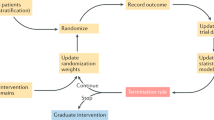
Abstract
Phase 3 randomized controlled trials (RCTs), while the gold standard for treatment efficacy and safety, are not always feasible, are expensive, can be prolonged and can be limited in generalizability. Other under-recognized sources of evidence can also help advance drug development. Basic science, proof-of-concept studies and early-phase RCTs can provide evidence regarding the potential for clinical benefit. Real-world evidence generated from registries or observational datasets can provide insights into the treatment of rare diseases that often pose a challenge for trial recruitment. Pragmatic trials embedded in healthcare systems can assess the treatment effects in clinical settings among patient populations sometimes excluded from trials. This Perspective discusses potential sources of evidence that may be used to complement explanatory phase 3 RCTs and to speed the development of new cardiovascular medications. Content is derived from the 19th Global Cardiovascular Clinical Trialists meeting (December 2022), involving clinical trialists, patients, clinicians, regulators, funders and industry representatives.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
No new data were generated or analyzed in support of this research.
References
Paraskevas, K. I., de Borst, G. J. & Veith, F. J. Why randomized controlled trials do not always reflect reality. J. Vasc. Surg. 70, 607–614 (2019).
Dang, A. Real-world evidence: a primer. Pharmaceut. Med. 37, 25–36 (2023).
Moore, T. J., Heyward, J., Anderson, G. & Alexander, G. C. Variation in the estimated costs of pivotal clinical benefit trials supporting the US approval of new therapeutic agents, 2015–2017: a cross-sectional study. BMJ Open 10, e038863 (2020).
Brown, M. L., Gersh, B. J., Holmes, D. R., Bailey, K. R. & Sundt, T. M. 3rd From randomized trials to registry studies: translating data into clinical information. Nat. Clin. Pract. Cardiovasc. Med. 5, 613–620 (2008).
Sharma, M., Nazareth, I. & Petersen, I. Observational studies of treatment effectiveness: worthwhile or worthless? Clin. Epidemiol. 11, 35–42 (2019).
Fanaroff, A. C. et al. Randomized trials versus common sense and clinical observation: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 76, 580–589 (2020).
Ruberman, W., Weinblatt, E., Goldberg, J. D., Frank, C. W. & Shapiro, S. Ventricular premature beats and mortality after myocardial infarction. N. Engl. J. Med. 297, 750–757 (1977).
Echt, D. S. et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N. Engl. J. Med. 324, 781–788 (1991).
Steinmetz, K. L. & Spack, E. G. The basics of preclinical drug development for neurodegenerative disease indications. BMC Neurol. 9, S2 (2009).
Seyhan, A. A. Lost in translation: the valley of death across preclinical and clinical divide—identification of problems and overcoming obstacles. Transl. Med. Comm. 4, 18 (2019).
Paul, S. M. et al. How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat. Rev. Drug Discov. 9, 203–214 (2010).
Humbert, M. et al. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur. Respir. J. 53, 1801887 (2019).
Yung, L. M. et al. ACTRIIA-Fc rebalances activin/GDF versus BMP signaling in pulmonary hypertension. Sci. Transl. Med. 12, eaaz5660 (2020).
Joshi, S. R. et al. Sotatercept analog improves cardiopulmonary remodeling and pulmonary hypertension in experimental left heart failure. Front. Cardiovasc. Med. 10, 1064290 (2023).
Humbert, M. et al. Sotatercept for the treatment of pulmonary arterial hypertension. N. Engl. J. Med. 384, 1204–1215 (2021).
Hoeper, M. M. et al. Phase 3 trial of sotatercept for treatment of pulmonary arterial hypertension. N. Engl. J. Med. 388, 1478–1490 (2023).
Mohs, R. C. & Greig, N. H. Drug discovery and development: role of basic biological research. Alzheimers Dement. 3, 651–657 (2017).
Khan, M. S. et al. Leveraging electronic health records to streamline the conduct of cardiovascular clinical trials. Eur. Heart J. 44, 1890–1909 (2023).
Dagenais, S., Russo, L., Madsen, A., Webster, J. & Becnel, L. Use of real-world evidence to drive drug development strategy and inform clinical trial design. Clin. Pharmacol. Ther. 111, 77–89 (2022).
Zhao, X., Iqbal, S., Valdes, I. L., Dresser, M. & Girish, S. Integrating real-world data to accelerate and guide drug development: a clinical pharmacology perspective. Clin. Transl. Sci. 15, 2293–2302 (2022).
Szymanski, P. et al. Real world evidence: perspectives from a European Society of Cardiology Cardiovascular Round Table with contribution from the European Medicines Agency. Eur. Heart J. Qual. Care Clin. Outcomes 9, 109–118 (2023).
Leclercq, C. et al. Wearables, telemedicine, and artificial intelligence in arrhythmias and heart failure: proceedings of the European Society of Cardiology Cardiovascular Round Table. Europace 24, 1372–1383 (2022).
Honig, P. K. The ‘coming of age’ of real-world evidence in drug development and regulation. Clin. Pharmacol. Ther. 111, 11–14 (2022). This editorial underscores the importance of RWE in medical research to inform clinical trial design, elucidate the natural history of disease, select and monitor endpoints, compare effectiveness, and assess treatment utilization patterns.
Flynn, R. et al. Marketing authorization applications made to the European Medicines Agency in 2018–2019: what was the contribution of real-world evidence? Clin. Pharmacol. Ther. 111, 90–97 (2022).
Bakker, E. et al. Contribution of real-world evidence in European Medicines Agency’s regulatory decision making. Clin. Pharmacol. Ther. 113, 135–151 (2023).
Purpura, C. A., Garry, E. M., Honig, N., Case, A. & Rassen, J. A. The role of real-world evidence in FDA-approved new drug and biologics license applications. Clin. Pharmacol. Ther. 111, 135–144 (2022). In an application to the US FDA, RWE was influential in 74% (n = 65 out of 88) of decisions, demonstrating that RWE is an essential part of a regulatory approval package.
Cave, A., Kurz, X. & Arlett, P. Real-world data for regulatory decision making: challenges and possible solutions for Europe. Clin. Pharmacol. Ther. 106, 36–39 (2019).
Mofid, S., Bolislis, W. R. & Kuhler, T. C. Real-world data in the postapproval setting as applied by the EMA and the US FDA. Clin. Ther. 44, 306–322 (2022).
Lund, L. H., Oldgren, J. & James, S. Registry-based pragmatic trials in heart failure: current experience and future directions. Curr. Heart Fail. Rep. 14, 59–70 (2017).
Doherty, D. A. et al. Registry randomised trials: a methodological perspective. BMJ Open 13, e068057 (2023).
Van Spall, H. G. C. et al. Knowledge to action: rationale and design of the Patient-Centered Care Transitions in Heart Failure (PACT-HF) stepped wedge cluster randomized trial. Am. Heart J. 199, 75–82 (2018).
Van Spall, H. G. C. et al. Effect of patient-centered transitional care services on clinical outcomes in patients hospitalized for heart failure: the PACT-HF randomized clinical trial. JAMA 321, 753–761 (2019).
Frobert, O. et al. Thrombus aspiration during ST-segment elevation myocardial infarction. N. Engl. J. Med. 369, 1587–1597 (2013).
Hess, C. N. et al. Embedding a randomized clinical trial into an ongoing registry infrastructure: unique opportunities for efficiency in design of the Study of Access site For Enhancement of Percutaneous Coronary Intervention for Women (SAFE-PCI for Women). Am. Heart J. 166, 421–428 (2013).
Chew, D. S., Whitelaw, S., Vaduganathan, M., Mark, D. B. & Van Spall, H. G. C. Patient-reported outcome measures in cardiovascular disease: an evidence map of the psychometric properties of health status instruments. Ann. Intern. Med. 175, 1431–1439 (2022).
Zannad, F. et al. Patient-reported outcome measures and patient engagement in heart failure clinical trials: multi-stakeholder perspectives. Eur. J. Heart. Fail. 25, 478–487 (2023).
Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 69, 89–95 (2001).
Temple, R. Are surrogate markers adequate to assess cardiovascular disease drugs? JAMA 282, 790–795 (1999). This review of surrogate endpoints from a member of the FDA provides a balanced look at the pros and cons of relying on such outcomes in clinical research.
Spertus, J. V. et al. Integrating quality of life and survival outcomes in cardiovascular clinical trials. Circ. Cardiovasc. Qual. Outcomes 12, e005420 (2019).
Weir, C. J. & Taylor, R. S. Informed decision-making: Statistical methodology for surrogacy evaluation and its role in licensing and reimbursement assessments. Pharm. Stat. 21, 740–756 (2022).
Fleming, T. R. & DeMets, D. L. Surrogate end points in clinical trials: are we being misled? Ann. Intern. Med. 125, 605–613 (1996).
FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) Resource (US FDA and National Institutes of Health, 2016).
Elliott, P. et al. Development, validation, and implementation of biomarker testing in cardiovascular medicine state-of-the-art: proceedings of the European Society of Cardiology-Cardiovascular Round Table. Cardiovasc. Res. 117, 1248–1256 (2021).
Bakker, E. et al. Biomarker qualification at the European Medicines Agency: a review of biomarker qualification procedures from 2008 to 2020. Clin. Pharmacol. Ther. 112, 69–80 (2022). A review of biomarkers qualified by the EMA between 2008 and 2020 found that only 13 out of 86 applications were approved, and of 9 efficacy biomarkers proposed as surrogate endpoints, none were qualified, demonstrating that this field continues to need more work.
Fleming, T. R. & Powers, J. H. Biomarkers and surrogate endpoints in clinical trials. Stat. Med. 31, 2973–2984 (2012).
Kim, M. S. et al. A draft map of the human proteome. Nature 509, 575–581 (2014).
Ridker, P. M., Rifai, N., Rose, L., Buring, J. E. & Cook, N. R. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N. Engl. J. Med. 347, 1557–1565 (2002).
Mohebi, R., McCarthy, C. P., Gaggin, H. K., van Kimmenade, R. R. J. & Januzzi, J. L. Inflammatory biomarkers and risk of cardiovascular events in patients undergoing coronary angiography. Am. Heart J. 252, 51–59 (2022).
Mohebi, R. et al. Inflammation across universal definition of heart failure stages: the CASABLANCA study. Eur. J. Heart Fail. 25, 152–160 (2022).
Ravera, A. et al. Distinct pathophysiological pathways in women and men with heart failure. Eur. J. Heart Fail. 24, 1532–1544 (2022).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Marwick, T. H., Cho, I., Hartaigh, B. O. & Min, J. K. Finding the gatekeeper to the cardiac catheterization laboratory: coronary CT angiography or stress testing? J. Am. Coll. Cardiol. 65, 2747–2756 (2015).
Figtree, G. A. et al. Noninvasive plaque imaging to accelerate coronary artery disease drug development. Circulation 146, 1712–1727 (2022).
Lee, S. E. et al. Effects of statins on coronary atherosclerotic plaques: the PARADIGM study. JACC Cardiovasc. Imaging 11, 1475–1484 (2018).
SCOT-HEART Investigators. et al. Coronary CT angiography and 5-year risk of myocardial infarction. N. Engl. J. Med. 379, 924–933 (2018). This trial demonstrated that CTCA added to standard care significantly lowered the rate of CV events at 5 years compared to standard care, suggesting the potential for plaque characteristics on CTCA to be used as surrogate endpoints.
Gulati, M. et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 144, e368–e454 (2021).
Knuuti, J. et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 41, 407–477 (2020).
Freeman, A. M. et al. Integrating coronary atherosclerosis burden and progression with coronary artery disease risk factors to guide therapeutic decision making. Am. J. Med. 136, 260–269 (2023).
Chang, H. J. et al. Coronary atherosclerotic precursors of acute coronary syndromes. J. Am. Coll. Cardiol. 71, 2511–2522 (2018).
Ferencik, M. et al. Use of high-risk coronary atherosclerotic plaque detection for risk stratification of patients with stable chest pain: a secondary analysis of the PROMISE randomized clinical trial. JAMA Cardiol. 3, 144–152 (2018).
Williams, M. C. et al. Coronary artery plaque characteristics associated with adverse outcomes in the SCOT-HEART study. J. Am. Coll. Cardiol. 73, 291–301 (2019).
van Rosendael, A. R. et al. Association of high-density calcified 1K plaque with risk of acute coronary syndrome. JAMA Cardiol. 5, 282–290 (2020).
van Rosendael, A. R. et al. Progression of whole-heart atherosclerosis by coronary CT and major adverse cardiovascular events. J. Cardiovasc. Comput. Tomogr. 15, 322–330 (2021).
Averbuch, T. et al. Applications of artificial intelligence and machine learning in heart failure. Eur. Heart J. Digit. Health 3, 311–322 (2022).
Greenberg, B., Adler, E., Campagnari, C. & Yagil, A. A machine learning risk score predicts mortality across the spectrum of left ventricular ejection fraction. Eur. J. Heart Fail. 23, 995–999 (2021).
Adler, E. D. et al. Improving risk prediction in heart failure using machine learning. Eur. J. Heart Fail. 22, 139–147 (2020).
Jering, K. S. et al. Improving clinical trial efficiency using a machine learning-based risk score to enrich study populations. Eur. J. Heart Fail. 24, 1418–1426 (2022).
Gevaert, A. B. et al. Clinical phenogroups are more effective than left ventricular ejection fraction categories in stratifying heart failure outcomes. ESC Heart Fail. 8, 2741–2754 (2021).
Acknowledgements
This article was generated from discussions at the 19th Global CVCT Forum held online in December 2022. The CVCT Forum is a strategic workshop for high-level dialog between clinical trialists, industry representatives, regulatory authorities and patients. The authors thank P. Lavigne and S. Portelance (unaffiliated, supported by the CVCT Forum) for contributions to writing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
H.G.C.V.S. reports grants from the Canadian Institutes of Health Research and the Heart and Stroke Foundation. A.B. reports employment, travel and/or meeting attendance support, and stock or stock options from Bristol Myers Squibb; and membership on the Board of Trustees (unpaid) for Coriell Medical Research Institute. J.M. reports participation on a medical advisory board for Arineta; employment (CEO) with Cleerly; and an equity interest in Cleerly. K.S. reports employment, travel and/or meeting attendance support, and stock or stock options from Olink Proteomics. F.Z. reports consulting fees from 89bio, Applied Therapeutics, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cardior Pharmaceuticals, Cellprothera, Cereno Scientific, CEVA, CVRx, Merck, Novartis, NovoNordisk, Owkin, Pfizer and Servier; honoraria for lectures from Bayer, Boehringer Ingelheim, CEVA, CVRx, Merck and Novartis; fees for participation on a data safety monitoring board or advisory board from Acceleron/Merck; and equity interests in G3 Pharmaceuticals, Cereno Pharmaceuticals, Cardiorenal, Eshmoun Clinical Research and CVCT. The other authors declare no competing interests. The views expressed in this article are the personal views of the authors and may not be understood or quoted as being made on behalf of or reflecting the position of the regulatory agency/agencies or organizations with which the authors are employed or affiliated.
Peer review
Peer review information
Nature Cardiovascular Research thanks Ulf Landmesser, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Van Spall, H.G.C., Bastien, A., Gersh, B. et al. The role of early-phase trials and real-world evidence in drug development. Nat Cardiovasc Res 3, 110–117 (2024). https://doi.org/10.1038/s44161-024-00420-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44161-024-00420-4