Abstract
Direct reprogramming of fibroblasts into induced cardiomyocytes (iCMs) holds great promise for heart regeneration. Although considerable progress has been made in understanding the transcriptional and epigenetic mechanisms of iCM reprogramming, its translational regulation remains largely unexplored. Here, we characterized the translational landscape of iCM reprogramming through integrative ribosome and transcriptomic profiling, and found extensive translatome repatterning during this process. Loss-of-function screening for translational regulators uncovered Y-box binding protein 1 (Ybx1) as a critical barrier to iCM induction. In a mouse model of myocardial infarction, removing Ybx1 enhanced in vivo reprogramming, resulting in improved heart function and reduced scar size. Mechanistically, Ybx1 depletion de-repressed the translation of its direct targets Srf and Baf60c, both of which mediated the effect of Ybx1 depletion on iCM generation. Furthermore, removal of Ybx1 allowed single-factor, Tbx5-mediated iCM conversion. In summary, the findings reveal a new layer of regulatory mechanism that controls cardiac reprogramming at the translational level.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files. Sequencing data are available from the NCBI Gene Expression Omnibus (GEO) under accession number GSE196127. All other data supporting the findings of this study are available from the corresponding author on reasonable request.
References
Ieda, M. et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 142, 375–386 (2010).
Qian, L. et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature 485, 593–598 (2012).
Song, K. et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature 485, 599–604 (2012).
Zhou, Y. et al. Bmi1 is a key epigenetic barrier to direct cardiac reprogramming. Cell Stem Cell 18, 382–395 (2016).
Liu, L. et al. Targeting Mll1 H3K4 methyltransferase activity to guide cardiac lineage specific reprogramming of fibroblasts. Cell Discov. 2, 1–11 (2016).
Zhou, H. et al. ZNF281 enhances cardiac reprogramming by modulating cardiac and inflammatory gene expression. Genes Dev. 31, 1770–1783 (2017).
Stone, N. R. et al. Context-specific transcription factor functions regulate epigenomic and transcriptional dynamics during cardiac reprogramming. Cell Stem Cell 25, 87–102 (2019).
Hashimoto, H. et al. Cardiac reprogramming factors synergistically activate genome-wide cardiogenic stage-specific enhancers. Cell Stem Cell 25, 69–86 (2019).
Wang, L. et al. Down-regulation of Beclin1 promotes direct cardiac reprogramming. Sci. Transl. Med. 12, eaay7856 (2020).
Garry, G. A. et al. The histone reader PHF7 cooperates with the SWI/SNF complex at cardiac super enhancers to promote direct reprogramming. Nat. Cell Biol. 23, 467–475 (2021).
Xie, Y., Liu, J. & Qian, L. Direct cardiac reprogramming comes of age: recent advance and remaining challenges. Semin. Cell Dev. Biol. 122, 37–43 (2021).
Christoforou, N. et al. Transcription factors MYOCD, SRF, Mesp1 and SMARCD3 enhance the cardio-inducing effect of GATA4, TBX5, and MEF2C during direct cellular reprogramming. PLoS ONE 8, e63577 (2013).
Addis, R. C. et al. Optimization of direct fibroblast reprogramming to cardiomyocytes using calcium activity as a functional measure of success. J. Mol. Cell. Cardiol. 60, 97–106 (2013).
Hirai, H., Katoku-Kikyo, N., Keirstead, S. A. & Kikyo, N. Accelerated direct reprogramming of fibroblasts into cardiomyocyte-like cells with the MyoD transactivation domain. Cardiovasc. Res. 100, 105–113 (2013).
Hirai, H. & Kikyo, N. Inhibitors of suppressive histone modification promote direct reprogramming of fibroblasts to cardiomyocyte-like cells. Cardiovasc. Res. 102, 188–190 (2014).
Liu, Z. et al. Single-cell transcriptomics reconstructs fate conversion from fibroblast to cardiomyocyte. Nature 551, 100–104 (2017).
Zhou, Y. et al. Single-cell transcriptomic analyses of cell fate transitions during human cardiac reprogramming. Cell Stem Cell 25, 149–164 (2019).
Wang, H., Yang, Y., Qian, Y., Liu, J. & Qian, L. Delineating chromatin accessibility re-patterning at single cell level during early stage of direct cardiac reprogramming. J. Mol. Cell. Cardiol. 162, 62–71 (2022).
Ingolia, N. T., Ghaemmaghami, S., Newman, J. R. S. & Weissman, J. S. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science 324, 218–223 (2009).
van Heesch, S. et al. The translational landscape of the human heart. Cell 178, 242–260 (2019).
Chothani, S. et al. Widespread translational control of fibrosis in the human heart by RNA-binding proteins. Circulation 140, 937–951 (2019).
Doroudgar, S. et al. Monitoring cell-type-specific gene expression using ribosome profiling in vivo during cardiac hemodynamic stress. Circ. Res. 125, 431–448 (2019).
Yan, Y. et al. The cardiac translational landscape reveals that micropeptides are new players involved in cardiomyocyte hypertrophy. Mol. Ther. 29, 2253–2267 (2021).
Chothani, S. et al. deltaTE: detection of translationally regulated genes by integrative analysis of Ribo-seq and RNA-seq data. Curr. Protoc. Mol. Biol. 129, e108 (2019).
Mammoto, A. & Ingber, D. E. Cytoskeletal control of growth and cell fate switching. Curr. Opin. Cell Biol. 21, 864–870 (2009).
De Bruin, R. G., Rabelink, T. J., Van Zonneveld, A. J. & Van Der Veer, E. P. Emerging roles for RNA-binding proteins as effectors and regulators of cardiovascular disease. Eur. Heart J. 38, 1380–1388 (2017).
Wang, Y., Arribas-Layton, M., Chen, Y., Lykke-Andersen, J. & Sen, G. L. DDX6 orchestrates mammalian progenitor function through the mRNA degradation and translation pathways. Mol. Cell 60, 118–130 (2015).
Kwon, E. et al. The RNA-binding protein YBX1 regulates epidermal progenitors at a posttranscriptional level. Nat. Commun. 9, 1734 (2018).
Evdokimova, V. et al. Translational activation of Snail1 and other developmentally regulated transcription factors by YB-1 promotes an epithelial-mesenchymal transition. Cancer Cell 15, 402–415 (2009).
El-Naggar, A. M. et al. Translational activation of HIF1α by YB-1 promotes sarcoma metastasis. Cancer Cell 27, 682–697 (2015).
Huang, P. et al. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature 475, 386–389 (2011).
Vierbuchen, T. et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463, 1035–1041 (2010).
Ackers-Johnson, M. et al. A simplified, Langendorff-free method for concomitant isolation of viable cardiac myocytes and nonmyocytes from the adult mouse heart. Circ. Res. 119, 909–920 (2016).
Feng, M. et al. YBX1 is required for maintaining myeloid leukemia cell survival by regulating BCL2 stability in an m6A-dependent manner. Blood 138, 71–85 (2021).
Mordovkina, D. et al. Y-box binding proteins in mRNP assembly, translation, and stability control. Biomolecules 10, 591 (2020).
Street, K. et al. Slingshot: cell lineage and pseudotime inference for single-cell transcriptomics. BMC Genomics 19, 477 (2018).
Boogerd, C. J. et al. Tbx20 is required in mid-gestation cardiomyocytes and plays a central role in atrial development. Circ. Res. 123, 428–442 (2018).
Yamakawa, H. et al. Fibroblast growth factors and vascular endothelial growth factor promote cardiac reprogramming under defined conditions. Stem Cell Reports 5, 1128–1142 (2015).
Tani, H. et al. Direct reprogramming improves cardiac function and reverses fibrosis in chronic myocardial infarction. Circulation 147, 223–238 (2023).
Evdokimova, V., Ovchinnikov, L. P. & Sorensen, P. H. B. Y-box binding protein 1: providing a new angle on translational regulation. Cell Cycle 5, 1143–1147 (2006).
Ovchinnikov, L. P., Skabkin, M. A., Ruzanov, P. V. & Evdokimova, V. M. Major mRNP proteins in the structural organization and function of mRNA in eukaryotic cells. Mol. Biol. 35, 462–471 (2001).
Evdokimova, V. et al. The major mRNA-associated protein YB-1 is a potent 5′ cap-dependent mRNA stabilizer. EMBO J. 20, 5491–5502 (2001).
Kumari, P. et al. An essential role for maternal control of Nodal signaling. eLife 2, e00683 (2013).
Zaucker, A. et al. Translational co-regulation of a ligand and inhibitor by a conserved RNA element. Nucleic Acids Res. 46, 104–119 (2018).
Lyabin, D. N. et al. YB-3 substitutes YB-1 in global mRNA binding. RNA Biol. 17, 487–499 (2020).
Balza, R. O. & Misra, R. P. Role of the serum response factor in regulating contractile apparatus gene expression and sarcomeric integrity in cardiomyocytes. J. Biol. Chem. 281, 6498–6510 (2006).
Lickert, H. et al. Baf60c is essential for function of BAF chromatin remodelling complexes in heart development. Nature 432, 107–112 (2004).
Lei, I., Liu, L., Sham, M. H. & Wang, Z. SWI/SNF in cardiac progenitor cell differentiation. J. Cell. Biochem. 114, 2437–2445 (2013).
Plotkin, J. B. Transcriptional regulation is only half the story. Molecular Systems Biology 6, 406 (2010).
Sauls, K. et al. Initiating events in direct cardiomyocyte reprogramming. Cell Rep. 22, 1913–1922 (2018).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Liao, Y., Smyth, G. K. & Shi, W. FeatureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Calviello, L. et al. Detecting actively translated open reading frames in ribosome profiling data. Nat. Methods 13, 165–170 (2016).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587 (2021).
Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2009).
Xie, Y. et al. MircroRNA-10b promotes human embryonic stem cell-derived cardiomyocyte proliferation via novel target gene LATS1. Mol. Ther. Nucleic Acids 19, 437–445 (2020).
Wang, L. et al. Stoichiometry of Gata4, Mef2c, and Tbx5 influences the efficiency and quality of induced cardiac myocyte reprogramming. Circ. Res. 116, 237–244 (2015).
Xie, Y. et al. Rare mutations of ADAM17 from TOFs induce hypertrophy in human embryonic stem cell-derived cardiomyocytes via HB-EGF signaling. Clin. Sci. 133, 225–238 (2019).
Wang, H. et al. Cross-lineage potential of Ascl1 uncovered by comparing diverse reprogramming regulatomes. Cell Stem Cell 29, 1491–1504 (2022).
Acknowledgements
We thank all of the members of the Qian and Liu laboratories for helpful discussions and their valuable input. We would also like to acknowledge the Flow Cytometry Core of the University of North Carolina at Chapel Hill (NIH National Cancer Institute (NCI) grant P30CA016086), Advanced Analytics Core, and High-Throughput Sequencing Facility (NIH NCI grant P30CA016086, NIH National Institute of Environmental Health Sciences (NIEHS) grant P30ES010126). This work was supported by American Heart Association (AHA) grant 20EIA35310348, NIH National Heart Lung and Blood Institute (NHLBI) grant R35HL155656 to L.Q., NIH NHLBI grants R01HL139976 and R01HL139880; AHA Established Investigator Award 20EIA35320128 to J.L.; NIMH grants S10MH124745 and S10OD026796 to Y.S.; and AHA grant 23POST1026377 to Y.X.
Author information
Authors and Affiliations
Contributions
Y.X. conceived the project, performed the experiments and wrote the manuscript with contributions from all other authors. Y.Y. and M.C. performed data analysis. C.S. prepared Ribo-seq and RNA-seq libraries. Q.W., D.N., H.W., C.N., B.K. and G.F. assisted with experiments and data analysis. T.W., S.L. and Y.S. assisted with MRI and related analysis. J.L. and L.Q. supervised the project, provided the funding and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
C.S. is an employee of EIRNA Bio. All other authors declare no competing interests.
Peer review
Peer review information
Nature Cardiovascular Research thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor Elisa Martini, in collaboration with the Nature Cardiovascular Research Team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended Data
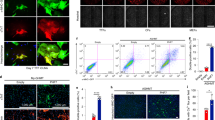
Extended Data Fig. 1 Data quality evaluation and shRNA screening identified Ybx1 as a barrier of iCM reprogramming.
(a) Representative ICC images for cTnT+ and αActinin+ cells on day 5 MGT-infected CFs. The experiments were repeated 3 biological times. (b) Bar plot showing the percentage of in-frame reads for all Ribo-seq libraries. (c) Pearson’s correlation analysis of Ribo-seq and RNA-seq data between two replicates of each time point. (d) Knockdown efficiency of indicated shRNA pools measured by RT-qPCR (n = 3 independent experiments). Expression values of each gene were normalized to those measured in shNT groups. (e) Histogram of normalized percentages of cTnT+ and αActinin+ cells from positive hits (n = 3 independent experiments). (f-g) Western blot and quantification of Ybx1 and αActinin expression at different points (D0, D6 and D12) during cardiac reprogramming (n = 3 independent experiments). Statistical significance was calculated using two-tailed t test for paired samples and one-way ANOVA with Tukey’s multiple comparisons test (adjusted P value) for multiple groups. Error bars indicate means ± SEM.
Extended Data Fig. 2 Depletion of Ybx1 increased cardiac reprogramming efficiency.
(a) Flow gating strategy: cells were gated on size selection (forward/side scatter), singlets (standard gating strategy) and antibody staining. (b) Knockdown efficiency of individual Ybx1 shRNA evaluated by RT-qPCR (n = 3 independent experiments). (c) Representative flow cytometry and quantification analyses of cTnT+ and αActinin+ cells in reprogramming cultures 10 days after infection with MGT and Ybx1 shRNAs virus, n = 3 independent experiments. (d) Quantification of Ybx1 knockdown efficiency by siRNAs in CFs (n = 3 independent experiments). (e-f) Representative ICC images and quantification data for cTnT+ and αActinin+ cells 10 days after transfection MGT-infected CFs with siRNA-Control or siRNA-Ybx1(n = 3 independent experiments). (g) Representative ICC images and quantification for cTnT+ and αMHC-GFP+ cells on MGT-infected MEFs with indicated shRNAs (n = 10, each data point represents an image window for each sample and the experiments were repeated at least 3 times independently). (h) Representative flow plots and quantification cTnT and αMHC-GFP cells on MGT-infected MEFs with indicated shRNAs (n = 3 independent experiments). (i) Representative ICC images and quantification for cTnT+ and αMHC-GFP+ cells on MGT-infected TTFs with indicated shRNAs (n = 10, each data point represents an image window for each sample and the experiments were repeated at least 3 times independently). (j) Representative flow plots and quantification cTnT and αMHC-GFP cells on MGT-infected TTFs with indicated shRNAs (n = 3 independent experiments). Statistical significance was calculated using two-tailed t test for paired samples and one-way ANOVA with Tukey’s multiple comparisons test (adjusted P value) for multiple groups. Error bars indicate means ± SEM.
Extended Data Fig. 3 Knockdown of YBX1 promoted human iCM reprogramming.
(a) Representative flow plots and quantification for cTnT+ and αActinin+ cells 12 days after human cardiac reprogramming cocktail and indicated shRNA transduction into H9F (n = 3 independent experiments). (b) Representative ICC images and quantification of human iCMs in shNT and shYBX1 reprogramming cultures (n = 10, each data point represents an image window for each sample and the experiments were repeated at least 3 times independently). (c) Quantification of the relative expression of a set of cardiac genes and fibroblast genes in indicated groups (n = 3 independent experiments). (d) Representative flow plots and quantification for cTnT+ and αActinin+ cells 12 days after human cardiac reprogramming cocktail and indicated shRNA transduction into hCFs (n = 3 independent experiments). (e) Representative ICC images and quantification of human iCMs in shNT and shYBX1 reprogramming cultures (n = 10), each data point represents an image window for each sample and the experiments were repeated at least 3 times independently. (f) Quantification of the relative expression of a set of cardiac genes and fibroblast genes in indicated groups (n = 3 independent experiments). (g) Schematics of hiCM (labeled with GFP) co-culture experiments with beating hiPSC-CMs. (h) Representative images and quantification of observed beating hiCMs after 1 month co-culture with hiPSC-CMs in indicated groups (n = 3 independent experiments). (i) Calcium dye tracing and quantification of co-cultured hiCMs exhibiting calcium transits in indicated groups (n = 3 independent experiments). Statistical significance was calculated using two-tailed t test for paired samples. Error bars indicate means ± SEM.
Extended Data Fig. 4 Ybx1 overexpression does not suppress iCM reprogramming and Ybx1 is required of iN and iHep reprogramming.
(a) Ybx1 overexpression level in CFs (n = 3 independent experiments). (b) Representative ICC images and quantification data for cTnT+ and αActinin+ cells 10 days after MGT and Ctr or Ybx1 infection on freshly isolated CFs (n = 3 independent experiments). (c) Quantification of cardiac gene expression in MGT-infected CFs with Ctr or Ybx1 by qRT-PCR (n = 3 independent experiments). (d) Representative flow plots and quantification data for cTnT+ and αActinin+ cells 10 days after MGT and Ctr or Ybx1 infection on freshly isolated CFs (n = 3 independent experiments). (e and h) Schematic depiction of experimental design using iHep or iN reprogramming cocktail and shRNAs. (f) Representative ICC images and quantification for Albumin+ cells on iHep reprogramming cocktail infected MEFs with indicated shRNAs (n = 5, each data point represents an image window for each sample and the experiments were repeated at least 3 times independently). (g) Quantification of hepatocyte-related gene expression by qRT-PCR (n = 3 independent experiments). (i) Representative ICC images and quantification for Tubb3+ cells on iN reprogramming cocktail infected MEFs with indicated shRNAs (n = 5, each data point represents an image window for each sample and the experiments were repeated at least 3 times independently). (j) Quantification of neuron-related gene expression by qRT-PCR (n = 3 independent experiments). Statistical significance was calculated using two-tailed t test for paired samples. Error bars indicate means ± SEM. Boxes indicate interquartile range (IQR; 25th and 75th percentiles); center line indicates median (50th percentile); whiskers indicate minimum to maximum.
Extended Data Fig. 5 Inducible knockdown of Ybx1 at different time points along reprogramming.
(a) Schematic of experimental design to determine the time window for Ybx1 knockdown by inducible shRNA. (b and d) Representative images and quantification FC in the percentage of cTnT+ and αActinin+ cells in inducible shNT or shYbx1 groups as described in (c) (n = 3 independent experiments). (c) qRT-PCR analysis of Ybx1 expression level in inducible shNT and shYbx1 group with or without Doxycycline (Dox) (n = 3 independent experiments). (c-d)Statistical significance was calculated using two-tailed t test for paired samples. Error bars indicate means ± SEM.
Extended Data Fig. 6 Evaluation of initial cardiac damage, assessment of cardiac function by echocardiography and characterization of the myocyte number of different experimental groups.
(a) Schematic of ELISA for detection cTnl level in serum. (b) Quantification of plasma cTnl level in indicated groups (n = 8 mice). (c-d) Representative images and quantification for TUNEL+ cells 1 day after MI in indicated groups (n = 4 mice). (e) Representative image of Evans blue staining. (f) Quantification of AAR and infarct size 1 day after MI in indicated groups (n = 3 mice). (g) EF and FS of mice in different groups were plotted over time staring from baseline measurements (n = 8 mice). (h) Schematics of adult CM isolation by Langendorff-free method after MI. (i) Representative image of the isolated CMs. (j) Quantification of total ventricle CM number in indicated groups (n = 4 mice). Statistical significance was calculated using two-tailed t test for paired samples and one-way ANOVA with Tukey’s multiple comparisons test (adjusted P value) for multiple groups. Error bars indicate means ± SEM.
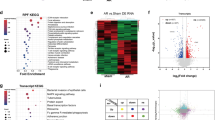
Extended Data Fig. 7 RIP-qPCR results of potential YBX1 binding targets.
(a-g) The distribution of YBX1-binding peaks within different gene regions from published YBX1 RIP-seq and RIP-qPCR results of potential YBX1 binding targets, Hey2 (a), Mesp1 (b), Gata6 (c), Tbx20 (d), Hand1 (e), Hand2 (f) and Nkx2.5 (g), n = 3 independent experiments. Data are shown as mean ± SEM. (h) Western blot and quantification of genes from A-G in shNT or shYbx1-infected CFs (n = 3 independent experiments). Statistical significance was calculated using two-tailed t test for paired samples. Error bars indicate means ± SEM.
Extended Data Fig. 8 Tbx5+shYbx1 treatment induced cardiac cells from human fibroblasts.
(a-b) Representative flow plots and quantification data for αActinin+ cells 12 days after reprogramming factor (TBX5) and indicated shRNAs infected hCFs (n = 3 independent experiments). (c) Representative image of hiCMs in TBX5 +shYBX1 cultures. (d) Quantification of cardiac gene expression in indicated groups (n = 3 independent experiments). Statistical significance was calculated using two-tailed t test for paired samples. Error bars indicate means ± SEM.
Extended Data Fig. 9 scRNA-seq replicates.
(a) UMAP visualization of Tbx5+shNT and Tbx5+shYbx1 replicates. (b-c) Proportion of cells in each of the cluster across replicates is shown for TBX5+shNT (b) and TBX5+shYbx1 (c).
Supplementary information
Supplementary Table 1
shRNA oligos
Supplementary Table 2
Primers for RT–qPCR
Supplementary Table 3
Primers for RIP–qPCR
Supplementary Data 1
Unprocessed blots
Source data
Source Data Fig. 1
Statistical Source Data
Source Data Fig. 2
Statistical Source Data
Source Data Fig. 3
Statistical Source Data
Source Data Fig. 4
Statistical Source Data
Source Data Fig. 5
Statistical Source Data
Source Data Fig. 6
Statistical Source Data
Source Data Fig. 7
Statistical Source Data
Source Data Fig. 8
Statistical Source Data
Source Data Extended Data Fig. 1
Statistical Source Data
Source Data Extended Data Fig. 2
Statistical Source Data
Source Data Extended Data Fig. 3
Statistical Source Data
Source Data Extended Data Fig. 4
Statistical Source Data
Source Data Extended Data Fig. 5
Statistical Source Data
Source Data Extended Data Fig. 6
Statistical Source Data
Source Data Extended Data Fig. 7
Statistical Source Data
Source Data Extended Data Fig. 8
Statistical Source Data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xie, Y., Wang, Q., Yang, Y. et al. Translational landscape of direct cardiac reprogramming reveals a role of Ybx1 in repressing cardiac fate acquisition. Nat Cardiovasc Res 2, 1060–1077 (2023). https://doi.org/10.1038/s44161-023-00344-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44161-023-00344-5
This article is cited by
-
Recent advances and future prospects in direct cardiac reprogramming
Nature Cardiovascular Research (2023)