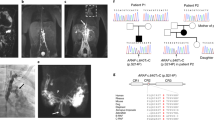
Abstract
Sirolimus, by targeting the mammalian target of rapamycin (mTOR) pathway, has demonstrated efficacy on lymphatic malformations (LMs) in adults and neonates. The current hypothesis is that the earlier the lesion is treated, the better it responds. This has prompted the idea that sirolimus administration might be efficacious to treat fetal LMs as well. Here we report a successful management of a cervicofacial fetal LM with sirolimus taken orally by the mother from the 22nd week of pregnancy until 2 weeks before planned delivery. Repeated cordocentesis recorded a 30% transplacental crossing of sirolimus. Continuation of sirolimus after birth allowed resection of the residual mass. We have followed the physical and neurological evolution of the child for 6 years since the fetal administration of sirolimus. We conclude that early administration of sirolimus during pregnancy with maternal serum monitoring may be proposed to high-risk fetal LMs in selected cases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data generated and analyzed during this study are included in the published article.
References
Perkins, J. A. et al. Clinical and radiographic findings in children with spontaneous lymphatic malformation regression. Otolaryngol. Head Neck Surg. 138, 772–777 (2008).
Connell, F. et al. Congenital vascular malformations: a series of five prenatally diagnosed cases. Am. J. Med. Genet. A 146, 2673–2680 (2008).
Zheng, J. W. et al. Treatment guideline for hemangiomas and vascular malformations of the head and neck. Head Neck 32, 1088–1098 (2010).
Johnson, A. B. & Richter, G. T. Surgical considerations in vascular malformations. Tech. Vasc. Interv. Radiol. 22, 100635 (2019).
Queisser, A., Seront, E., Boon, L. M. & Vikkula, M. Genetic basis and therapies for vascular anomalies. Circ. Res. 129, 155–173 (2021).
Seront, E., Van Damme, A., Boon, L. M. & Vikkula, M. Rapamycin and treatment of venous malformations. Curr. Opin. Hematol. 26, 185–192 (2019).
Adams, D. M. et al. Efficacy and safety of sirolimus in the treatment of complicated vascular anomalies. Pediatrics 137, e20153257 (2016).
Hammer, J. et al. Sirolimus is efficacious in treatment for extensive and/or complex slow-flow vascular malformations: a monocentric prospective phase II study. Orphanet J. Rare Dis. 13, 191 (2018).
Raveh, E., de Jong, A. L., Taylor, G. P. & Forte, V. Prognostic factors in the treatment of lymphatic malformations. Arch. Otolaryngol. Head Neck Surg. 123, 1061–1065 (1997).
Park, H., Chang, C. S., Choi, S. J., Oh, S. Y. & Roh, C. R. Sirolimus therapy for fetal cardiac rhabdomyoma in a pregnant woman with tuberous sclerosis. Obstet. Gynecol. Sci. 62, 280–284 (2019).
Dagge, A. et al. Fetal tuberous sclerosis: sirolimus for the treatment of fetal rhabdomyoma. Fetal Pediatr. Pathol. 4, 800–806 (2022).
Costanzo, M. R. et al. International Society of Heart and Lung Transplantation Guidelines. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J. Heart Lung Transplant. 29, 914–956 (2010).
Livingston, J. et al. Fetal therapy using rapamycin for a rapidly enlarging, obstructive, cervical lymphatic malformation: a case report. Prenat. Diagn. 41, 884–887 (2021).
Barnes, B. T. et al. Maternal sirolimus therapy for fetal cardiac rhabdomyomas. N. Engl. J. Med. 378, 1844–1845 (2018).
Tanvisut, R. et al. Cordocentesis-associated fetal loss and risk factors: single-center experience with 6650 cases. Ultrasound Obstet. Gynecol. 56, 664–671 (2020).
Zwiers, C., van Kamp, I., Oepkes, D. & Lopriore, E. Intrauterine transfusion and non-invasive treatment options for hemolytic disease of the fetus and newborn—review on current management and outcome. Expert Rev. Hematol. 10, 337–344 (2017).
Shen, L. et al. Pregnancy after the diagnosis of lymphangioleiomyomatosis (LAM). Orphanet J. Rare Dis. 16, 133 (2021).
Tshering, S., Dorji, N., Youden, S. & Wangchuk, D. Maternal sirolimus therapy and fetal growth restriction. Arch. Clin. Cases 8, 19–24 (2021).
Vachon-Marceau, C. et al. In-utero treatment of large symptomatic rhabdomyoma with sirolimus. Ultrasound Obstet. Gynecol. 53, 420–421 (2019).
Acknowledgements
All authors of this publication are members of the Vascular Anomaly Working Group (VASCA WG) of the European Reference Network for Rare Multisystemic Vascular Diseases (VASCERN) (project ID: 769036). We thank the parents of the patient for consenting to having their child’s history and photographs published. The studies in the groups of M.V. and L.M.B. were financially supported by the Fonds de la Recherche Scientifique (FNRS grants T.0247.19 (to M.V.) and T.0146.16 and P.C013.20 (to L.M.B.)); the Fund Generet managed by the King Baudouin Foundation (grant 2018-J1810250-211305) (to M.V.); the Walloon Region through the FRFS-WELBIO strategic research program (WELBIO-CR-2019C-06) (to M.V.); the Leducq Foundation Networks of Excellence Program grant ‘ReVAMP’ (LFCR grant 21CVD03); and the European Union’s Horizon 2020 Research and Innovation Program Theralymph under grant agreement 874708. They were also supported by a Pierre M. fellowship (M.V.). The authors are grateful to D. Adams (Children’s Hospital of Philadelphia) for expert review and suggestions for the manuscript; the nurse coordinator, O. Debue (Center for Vascular Anomalies, Saint-Luc University Hospital, UCLouvain), for excellent assistance in patient follow-up; and P. Dresse (Human Molecular Genetics, de Duve Institute, UCLouvain) and L. Huybrechts (Center for Vascular Anomalies, Saint-Luc University Hospital, UCLouvain) for their skilled secretarial help.
Author information
Authors and Affiliations
Contributions
E.S., A.V.D, M.V and L.M.B. conceived and designed the study. M.V. and L.M.B. obtained funds for the research. E.S., A.V.D., C.d.T., J.M.B., P.B. and L.M.B. followed the pregnancy. P.C. and D.D. analyzed the radiological data. B.L., S.S., F.V., C.P. and L.M.B. were involved in the clinical and surgical care of the child after birth. N.R. was in charge of the genetic analyses. All authors analyzed the data and contributed to writing and reviewing the manuscript. Each author approved the submitted manuscript and agreed both to be personally accountable for their own contributions and to ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved and the resolution documented in the literature.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cardiovascular Research thanks Bas Verhoeven, Benjamin T. Barnes and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
(1) Detailed informed consent. (2) Patient perspective.
Source data
Source Data Fig. 2.
Numerical source data for Fig. 2.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Seront, E., Biard, J.M., Van Damme, A. et al. A case report of sirolimus use in early fetal management of lymphatic malformation. Nat Cardiovasc Res 2, 595–599 (2023). https://doi.org/10.1038/s44161-023-00280-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44161-023-00280-4