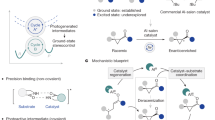
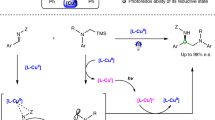
Abstract
This Review aims to highlight the developments in the field of visible-light-mediated enantioselective photocatalysis utilizing bifunctional organic photocatalysts by giving an overview of the existing hybrid structures and a summary of the reactions in which they have been employed. Organic bifunctional structures in this context are defined as the combination of organic photocatalyst moieties that can be activated by visible light with chiral catalysts responsible for enantioselective induction. An emphasis is put on comparing hybrid systems with dual-catalytic versions featuring two discrete catalysts, when applicable, while also pointing out limitations of current designs. Furthermore, mechanistic considerations are discussed, along with possible future directions in the development of new hybrid catalysts and the reactions that might benefit from these bifunctional structures.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Bellotti, P., Huang, H.-M., Faber, T. & Glorius, F. Photocatalytic late-stage C–H functionalization. Chem. Rev. 123, 4237–4352 (2023).
Chan, A. Y. et al. Metallaphotoredox: the merger of photoredox and transition metal catalysis. Chem. Rev. 122, 1485–1542 (2022).
Hojo, R., Polgar, A. M. & Hudson, Z. M. Thermally activated delayed fluorescence sensitizers as organic and green alternatives in energy-transfer photocatalysis. ACS Sustain. Chem. Eng. 10, 9665–9678 (2022).
Rigotti, T. & Alemán, J. Visible light photocatalysis—from racemic to asymmetric activation strategies. Chem. Commun. 56, 11169–11190 (2020).
Marzo, L., Pagire, S. K., Reiser, O. & König, B. Visible-light photocatalysis: does it make a difference in organic synthesis? Angew. Chem. Int. Ed. 57, 10034–10072 (2018).
Strieth-Kalthoff, F., James, M. J., Teders, M., Pitzer, L. & Glorius, F. Energy transfer catalysis mediated by visible light: principles, applications, directions. Chem. Soc. Rev. 47, 7190–7202 (2018).
Twilton, J. et al. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 1, 0052 (2017).
Romero, N. A. & Nicewicz, D. A. Organic photoredox catalysis. Chem. Rev. 116, 10075–10166 (2016).
Prier, C. K., Rankic, D. A. & MacMillan, D. W. C. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113, 5322–5363 (2013).
Großkopf, J., Kratz, T., Rigotti, T. & Bach, T. Enantioselective photochemical reactions enabled by triplet energy transfer. Chem. Rev. 122, 1626–1653 (2022).
Verma, R. et al. Recent trends in photocatalytic enantioselective reactions. Top. Curr. Chem. 380, 48 (2022).
Hoffmann, N. Enantioselective synthesis of heterocyclic compounds using photochemical reactions. Photochem. Photobiol. Sci. 20, 1657–1674 (2021).
Prentice, C., Morrisson, J., Smith, A. D. & Zysman-Colman, E. Recent developments in enantioselective photocatalysis. Beilstein J. Org. Chem. 16, 2363–2441 (2020).
Saha, D. Catalytic enantioselective radical transformations enabled by visible light. Chem. Asian J. 15, 2129–2152 (2020).
Jiang, C., Chen, W., Zheng, W.-H. & Lu, H. Advances in asymmetric visible-light photocatalysis, 2015–2019. Org. Biomol. Chem. 17, 8673–8689 (2019).
Silvi, M. & Melchiorre, P. Enhancing the potential of enantioselective organocatalysis with light. Nature 554, 41–49 (2018).
Zou, Y.-Q., Hörmann, F. M. & Bach, T. Iminium and enamine catalysis in enantioselective photochemical reactions. Chem. Soc. Rev. 47, 278–290 (2018).
Brenninger, C., Jolliffe, J. D. & Bach, T. Chromophore activation of α,β-unsaturated carbonyl compounds and its application to enantioselective photochemical reactions. Angew. Chem. Int. Ed. 57, 14338–14349 (2018).
Brimioulle, R., Lenhart, D., Maturi, M. M. & Bach, T. Enantioselective catalysis of photochemical reactions. Angew. Chem. Int. Ed. 54, 3872–3890 (2015).
Meggers, E. Asymmetric catalysis activated by visible light. Chem. Commun. 51, 3290–3301 (2015).
Roy, S., Paul, H. & Chatterjee, I. Light-mediated aminocatalysis: the dual-catalytic ability enabling new enantioselective route. Eur. J. Org. Chem. 2022, e202200446 (2022).
Guo, F., Wang, H., Ye, X. & Tan, C.-H. Advanced synthesis using photocatalysis involved dual catalytic system. Eur. J. Org. Chem. 2022, e202200326 (2022).
Yao, W., Bazan-Bergamino, E. A. & Ngai, M.-Y. Asymmetric photocatalysis enabled by chiral organocatalysts. ChemCatChem 14, e202101292 (2022).
Mondal, S. et al. Enantioselective radical reactions using chiral catalysts. Chem. Rev. 122, 5842–5976 (2022).
Genzink, M. J., Kidd, J. B., Swords, W. B. & Yoon, T. P. Chiral photocatalyst structures in asymmetric photochemical synthesis. Chem. Rev. 122, 1654–1716 (2022).
Huang, X. & Meggers, E. Asymmetric photocatalysis with bis-cyclometalated rhodium complexes. Acc. Chem. Res. 52, 833–847 (2019).
Rao, M., Wu, W. & Yang, C. Recent progress on the enantioselective excited-state photoreactions by pre-arrangement of photosubstrate(s). Green Synth. Catal. 2, 131–144 (2021).
Morimoto, M. et al. Advances in supramolecular host-mediated reactivity. Nat. Catal. 3, 969–984 (2020).
Crini, G. Review: a history of cyclodextrins. Chem. Rev. 114, 10940–10975 (2014).
Page, C. G. et al. Quaternary charge-transfer complex enables photoenzymatic intermolecular hydroalkylation of olefins. J. Am. Chem. Soc. 143, 97–102 (2021).
Sandoval, B. A. et al. Photoenzymatic reductions enabled by direct excitation of flavin-dependent ‘ene’-reductases. J. Am. Chem. Soc. 143, 1735–1739 (2021).
Black, M. J. et al. Asymmetric redox-neutral radical cyclization catalysed by flavin-dependent ‘ene’-reductases. Nat. Chem. 12, 71–75 (2020).
Biegasiewicz, K. F. et al. Photoexcitation of flavoenzymes enables a stereoselective radical cyclization. Science 364, 1166–1169 (2019).
Schirmer, T. E. & König, B. Ion-pairing catalysis in stereoselective, light-induced transformations. J. Am. Chem. Soc. 144, 19207–19218 (2022).
Dexter, D. L. A theory of sensitized luminescence in solids. J. Chem. Phys. 21, 836–850 (1953).
Alonso, R. & Bach, T. A chiral thioxanthone as an organocatalyst for enantioselective [2+2] photocycloaddition reactions induced by visible light. Angew. Chem. Int. Ed. 53, 4368–4371 (2014).
Cauble, D. F., Lynch, V. & Krische, M. J. Studies on the enantioselective catalysis of photochemically promoted transformations: ‘sensitizing receptors’ as chiral catalysts. J. Org. Chem. 68, 15–21 (2003).
Burg, F. & Bach, T. Lactam hydrogen bonds as control elements in enantioselective transition-metal-catalyzed and photochemical reactions. J. Org. Chem. 84, 8815–8836 (2019).
Maturi, M. M. & Bach, T. Enantioselective catalysis of the intermolecular [2+2] photocycloaddition between 2-pyridones and acetylenedicarboxylates. Angew. Chem. Int. Ed. 53, 7661–7664 (2014).
Müller, C. et al. Enantioselective intramolecular [2+2]-photocycloaddition reactions of 4-substituted quinolones catalyzed by a chiral sensitizer with a hydrogen-bonding motif. J. Am. Chem. Soc. 133, 16689–16697 (2011).
Müller, C., Bauer, A. & Bach, T. Light-driven enantioselective organocatalysis. Angew. Chem. Int. Ed. 48, 6640–6642 (2009).
Bauer, A., Westkämper, F., Grimme, S. & Bach, T. Catalytic enantioselective reactions driven by photoinduced electron transfer. Nature 436, 1139–1140 (2005).
Tröster, A., Alonso, R., Bauer, A. & Bach, T. Enantioselective intermolecular [2+2] photocycloaddition reactions of 2(1H)-quinolones induced by visible light irradiation. J. Am. Chem. Soc. 138, 7808–7811 (2016).
Li, X., Christian, J. & Bach, T. Visible-light-mediated enantioselective photoreactions of 3-alkylquinolones with 4-O-tethered alkenes and allenes. Org. Lett. 22, 3618–3622 (2020).
Li, X., Großkopf, J., Jandl, C. & Bach, T. Enantioselective, visible light mediated aza Paternò-Büchi reactions of quinoxalinones. Angew. Chem. Int. Ed. 60, 2684–2688 (2021).
Hölzl-Hobmeier, A. et al. Catalytic deracemization of chiral allenes by sensitized excitation with visible light. Nature 564, 240–243 (2018).
Plaza, M., Jandl, C. & Bach, T. Photochemical deracemization of allenes and subsequent chirality transfer. Angew. Chem. Int. Ed. 59, 12785–12788 (2020).
Plaza, M., Großkopf, J., Breitenlechner, S., Bannwarth, C. & Bach, T. Photochemical deracemization of primary allene amides by triplet energy transfer: a combined synthetic and theoretical study. J. Am. Chem. Soc. 143, 11209–11217 (2021).
Tröster, A., Bauer, A., Jandl, C. & Bach, T. Enantioselective visible-light-mediated formation of 3-cyclopropylquinolones by triplet-sensitized deracemization. Angew. Chem. Int. Ed. 58, 3538–3541 (2019).
Li, X. et al. Photochemically induced ring opening of spirocyclopropyl oxindoles: evidence for a triplet 1,3 diradical intermediate and deracemization by a chiral sensitizer. Angew. Chem. Int. Ed. 59, 21640–21647 (2020).
Kratz, T. et al. Photochemical deracemization of chiral alkenes via triplet energy transfer. J. Am. Chem. Soc. 144, 10133–10138 (2022).
Nikitas, N. F., Gkizis, P. L. & Kokotos, C. G. Thioxanthone: a powerful photocatalyst for organic reactions. Org. Biomol. Chem. 19, 5237–5253 (2021).
Zhou, T.-P., Zhong, F., Wu, Y. & Liao, R.-Z. Regioselectivity and stereoselectivity of intramolecular [2+2] photocycloaddition catalyzed by chiral thioxanthone: a quantum chemical study. Org. Biomol. Chem. 19, 1532–1540 (2021).
Yang, Y., Wen, Y., Dang, Z. & Yu, H. Mechanistic investigation of visible-light-induced intermolecular [2+2] photocycloaddition catalyzed with chiral thioxanthone. J. Phys. Chem. A 121, 4552–4559 (2017).
Murov, S. L., Carmichael, I. & Hug, G. L. Handbook of Photochemistry 3rd edn, 152, 197 (CRC, 2006).
Ding, W. et al. Bifunctional photocatalysts for enantioselective aerobic oxidation of β‑ketoesters. J. Am. Chem. Soc. 139, 63–66 (2017).
Yin, H. et al. Asymmetric bis(oxazoline)–Ni(II) catalyzed α-hydroxylation of cyclic β-keto esters under visible light. Org. Biomol. Chem. 19, 6588–6592 (2021).
Tang, X.-F. et al. Bifunctional metal-free photo-organocatalysts for enantioselective aerobic oxidation of β-dicarbonyl compounds. Tetrahedron 74, 3624–3633 (2018).
Tang, X.-F. et al. Visible-light-driven enantioselective aerobic oxidation of β-dicarbonyl compounds catalyzed by cinchona-derived phase transfer catalysts in batch and semi-flow. Adv. Synth. Catal. 361, 5245–5252 (2019).
Tang, X.-F. et al. Enantioselective photooxygenation of β-dicarbonyl compounds in batch and flow photomicroreactors. Org. Biomol. Chem. 17, 7938–7942 (2019).
Wang, Y. et al. A series of cinchona-derived N‑oxide phase-transfer catalysts: application to the photo-organocatalytic enantioselective α‑hydroxylation of β‑dicarbonyl compounds. J. Org. Chem. 81, 7042–7050 (2016).
Wang, Y. et al. Photo-organocatalytic enantioselective α-hydroxylation of β-keto esters and β-keto amides with oxygen under phase transfer catalysis. Green Chem. 18, 5493–5499 (2016).
Fischer, J., Mele, L., Serier-Brault, H., Nun, P. & Coeffard, V. Controlling photooxygenation with a bifunctional quinine-BODIPY catalyst: towards asymmetric hydroxylation of β-dicarbonyl compounds. Eur. J. Org. Chem. 2019, 6352–6358 (2019).
Mayr, F., Mohr, L.-M., Rodriguez, E. & Bach, T. Synthesis of chiral thiourea-thioxanthone hybrids. Synthesis 49, 5238–5250 (2017).
Vallavoju, N. et al. Organophotocatalysis: insights into the mechanistic aspects of thiourea-mediated intermolecular [2+2] photocycloadditions. Angew. Chem. Int. Ed. 55, 5446–5451 (2016).
Vallavoju, N., Selvakumar, S., Jockusch, S., Sibi, M. P. & Sivaguru, J. Enantioselective organo-photocatalysis mediated by atropisomeric thiourea derivatives. Angew. Chem. Int. Ed. 53, 5604–5608 (2014).
Vallavoju, N. et al. Evaluating thiourea architecture for intramolecular [2+2] photocycloaddition of 4-alkenylcoumarins. Adv. Synth. Catal. 356, 2763–2768 (2014).
Rigotti, T. et al. A bifunctional photoaminocatalyst for the alkylation of aldehydes: design, analysis, and mechanistic studies. ACS Catal. 8, 5928–5940 (2018).
Nicewicz, D. A. & MacMillan, D. W. C. Merging photoredox catalysis with organocatalysis: the direct asymmetric alkylation of aldehydes. Science 322, 77–80 (2008).
Gualandi, A. et al. Organocatalytic enantioselective alkylation of aldehydes with [Fe(bpy)3]Br2 catalyst and visible light. ACS Catal. 5, 5927–5931 (2015).
Fidaly, K. et al. Visible light photoredox organocatalysis: a fully transition metal-free direct asymmetric α-alkylation of aldehydes. Green Chem. 14, 1293–1297 (2012).
Neumann, M., Füldner, S., König, B. & Zeitler, K. Metal-free, cooperative asymmetric organophotoredox catalysis with visible light. Angew. Chem. Int. Ed. 50, 951–954 (2011).
Pecho, F. et al. A thioxanthone sensitizer with a chiral phosphoric acid binding site: properties and applications in visible light-mediated cycloadditions. Chem. Eur. J. 26, 5190–5194 (2020).
Lyu, J., Claraz, A., Vitale, M. R., Allain, C. & Masson, G. Preparation of chiral photosensitive organocatalysts and their application for the enantioselective synthesis of 1,2-diamines. J. Org. Chem. 85, 12843–12855 (2020).
Lyu, J. et al. Syntheses of new chiral chimeric photoorganocatalysts. RSC Adv. 11, 36663–36669 (2021).
Takagi, R. & Tanimoto, T. Enantioselective [2+2] photocycloaddition of quinolone using a C1-symmetric chiral phosphoric acid as a visible-light photocatalyst. Org. Biomol. Chem. 20, 3940–3947 (2022).
Levitre, G. et al. Combining organocatalysis and photoredox catalysis: an asymmetric synthesis of chiral β-amino α-substituted tryptamines. ChemCatChem 11, 5723–5727 (2019).
Dumoulin, A., Bernadat, G. & Masson, G. Enantioselective three-component amination of enecarbamates enables the synthesis of structurally complex small molecules. J. Org. Chem. 82, 1775–1789 (2017).
Dumoulin, A., Lalli, C., Retailleau, P. & Masson, G. Catalytic, highly enantioselective, direct amination of enecarbamates. Chem. Commun. 51, 5383–5386 (2015).
Pecho, F. et al. Enantioselective [2+2] Photocycloaddition via iminium ions: catalysis by a sensitizing chiral Brønsted acid. J. Am. Chem. Soc. 143, 9350–9354 (2021).
Rolka, A. B., Archipowa, N., Kutta, R. J., Toste, F. D. & König, B. Hybrid catalysts for enantioselective photo-phosphoric acid catalysis. J. Org. Chem. 88, 6509–6522 (2023).
Speckmeier, E., Fischer, T. G. & Zeitler, K. A toolbox approach to construct broadly applicable metal-free catalysts for photoredox chemistry: deliberate tuning of redox potentials and importance of halogens in donor–acceptor cyanoarenes. J. Am. Chem. Soc. 140, 15353–15365 (2018).
Luo, J. & Zhang, J. Donor-acceptor fluorophores for visible-light-promoted organic synthesis: photoredox/Ni dual catalytic C(sp3)-C(sp2) cross-coupling. ACS Catal. 6, 873–877 (2016).
Sherbrook, E. M. et al. Chiral Brønsted acid-controlled intermolecular asymmetric [2+2] photocycloadditions. Nat. Commun. 12, 5735 (2021).
Sherbrook, E. M., Jung, H., Cho, D., Baik, M.-H. & Yoon, T. P. Brønsted acid catalysis of photosensitized cycloadditions. Chem. Sci. 11, 856–861 (2020).
Liang, D., Chen, J.-R., Tan, L.-P., He, Z.-W. & Xiao, W.-J. Catalytic asymmetric construction of axially and centrally chiral heterobiaryls by Minisci reaction. J. Am. Chem. Soc. 144, 6040–6049 (2022).
Proctor, R. S. J., Davis, H. J. & Phipps, R. J. Catalytic enantioselective Minisci-type addition to heteroarenes. Science 360, 419–422 (2018).
Sauvé, E. R., Mayder, D. M. M., Kamal, S., Oderinde, M. S. & Hudson, Z. M. An imidazoacridine-based TADF material as an effective organic photosensitizer for visible-light-promoted [2+2] cycloaddition. Chem. Sci. 13, 2296–2302 (2022).
Sauvé, E. R., Paeng, J., Yamaguchi, S. & Hudson, Z. M. Donor–acceptor materials exhibiting thermally activated delayed fluorescence using a planarized N-phenylbenzimidazole acceptor. J. Org. Chem. 85, 108–117 (2020).
Uoyama, H., Goushi, K., Shizu, K., Nomura, H. & Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 492, 234–238 (2012).
Bryden, M. A. & Zysman-Colman, E. Organic thermally activated delayed fluorescence (TADF) compounds used in photocatalysis. Chem. Soc. Rev. 50, 7587–7680 (2021).
Shang, T.-Y. et al. Recent advances of 1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (4CzIPN) in photocatalytic transformations. Chem. Commun. 55, 5408–5419 (2019).
Lu, J. et al. Donor-acceptor fluorophores for energy-transfer-mediated photocatalysis. J. Am. Chem. Soc. 140, 13719–13725 (2018).
Srivastava, V., Singh, P. K., Srivastava, A., Sinha, S. & Singh, P. P. Recent advances of dicyanopyrazine (DPZ) in photoredox catalysis. Photochem 1, 237–246 (2021).
Zhao, Y. et al. Dicyanopyrazine-derived push–pull chromophores for highly efficient photoredox catalysis. RSC Adv. 4, 30062–30067 (2014).
Bureš, F. et al. Structure-property relationships and nonlinear optical effects in donor-substituted dicyanopyrazine-derivedpush–pull chromophores with enlarged and varied π-linkers.Eur. J. Org. Chem. 2012, 529–538 (2012).
Bobo, M. V., Kuchta, J. J. III & Vannucci, A. K. Recent advancements in the development of molecular organic photocatalysts. Org. Biomol. Chem. 19, 4816–4834 (2021).
Parmar, D., Sugiono, E., Raja, S. & Rueping, M. Complete field guide to asymmetric BINOL-phosphate derived Brønsted acid and metal catalysis: history and classification by mode of activation: Brønsted acidity, hydrogen bonding, ion pairing, and metal phosphates. Chem. Rev. 114, 9047–9153 (2014).
Hou, X.-Q. & Du, D.-M. Recent advances in squaramide-catalyzed asymmetric Mannich reactions. Adv. Synth. Catal. 362, 4487–4512 (2020).
Doyle, A. G. & Jacobsen, E. N. Small-molecule H-bond donors in asymmetric catalysis. Chem. Rev. 107, 5713–5743 (2007).
Wang, J., Lv, X. & Jiang, Z. Visible-light-mediated photocatalytic deracemization. Chem. Eur. J. 29, e20220402 (2023).
Neel, A. J., Hehn, J. P., Tripet, P. F. & Toste, F. D. Asymmetric cross-dehydrogenative coupling enabled by the design and application of chiral triazole-containing phosphoric acids. J. Am. Chem. Soc. 135, 14044–14047 (2013).
Liles, J. P. et al. Data science enables the development of a new class of chiral phosphoric acid catalysts. Chem 9, 1518–1537 (2023).
Crawford, J. M., Kingston, C., Toste, F. D. & Sigman, M. S. Data science meets physical organic chemistry. Acc. Chem. Res. 54, 3136–3148 (2021).
Werth, J. & Sigman, M. S. Connecting and analyzing enantioselective bifunctional hydrogen bond donor catalysis using data science tools. J. Am. Chem. Soc. 142, 16382–16391 (2020).
Reid, J. P. & Sigman, M. S. Holistic prediction of enantioselectivity in asymmetric catalysis. Nature 571, 343–348 (2019).
Zheng, J. et al. Enantioselective intermolecular excited-state photoreactions using a chiral Ir triplet sensitizer: separating association from energy transfer in asymmetric photocatalysis. J. Am. Chem. Soc. 141, 13625–13634 (2019).
Acknowledgements
A.B.R. thanks J. S. Tracy (University of West Florida) for helpful discussions and proofreading of the manuscript.
Author information
Authors and Affiliations
Contributions
A.B.R. wrote the manuscript. B.K. contributed to the discussion and helped revise the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Qing-Wei Meng and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Peter Seavill, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rolka, A.B., König, B. Bifunctional organic photocatalysts for enantioselective visible-light-mediated photocatalysis. Nat. Synth 2, 913–925 (2023). https://doi.org/10.1038/s44160-023-00398-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-023-00398-0