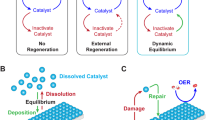
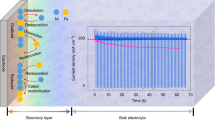
Abstract
The oxygen evolution reaction (OER) balances the hydrogen evolution reaction when splitting water into green hydrogen and oxygen with renewable electricity. Oxygen evolution occurs at potentials at which the pre-catalysts undergo transformations into complex and disordered OER-active oxides. Traditional synthetic methods and material compositions have resulted in an enormous variety of OER pre-catalysts, yet the most active of them are forced into a few prevailing oxyhydroxide or amorphous oxide phases under operation. As a result, practically relevant catalyst activity and stability have remained unchanged for decades. Here we discuss the need to develop new theory in synergy with operando electrochemical and spectroscopic characterization to take advantage of the effects of an applied electrochemical potential gradient on the chemical composition and atomic surface structure, the hydroxide ion intercalation and other interfacial ionics. Additionally, we highlight new synthesis methods and material compositions that might translate control over the pre-catalyst into control over the active oxide phase, as well as new methods of modifying active oxides during operation.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
McCrory, C. C. L. et al. Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J. Am. Chem. Soc. 137, 4347–4357 (2015).
Kibsgaard, J. & Chorkendorff, I. Considerations for the scaling-up of water splitting catalysts. Nat. Energy 4, 430–433 (2019).
Binninger, T. et al. Thermodynamic explanation of the universal correlation between oxygen evolution activity and corrosion of oxide catalysts. Sci. Rep. 5, 12167 (2015).
Danilovic, N. et al. Activity-stability trends for the oxygen evolution reaction on monometallic oxides in acidic environments. J. Phys. Chem. Lett. 5, 2474–2478 (2014).
Geiger, S. et al. The stability number as a metric for electrocatalyst stability benchmarking. Nat. Catal. 1, 508–515 (2018).
Fabbri, E. et al. Dynamic surface self-reconstruction is the key of highly active perovskite nano-electrocatalysts for water splitting. Nat. Mater. 16, 925–931 (2017).
Kasian, O., Grote, J., Geiger, S., Cherevko, S. & Mayrhofer, K. J. J. The common intermediates of oxygen evolution and dissolution reactions during water electrolysis on iridium. Angew. Chem. Int. Ed. 57, 2488–2491 (2018).
Chung, D. Y. et al. Dynamic stability of active sites in hydr(oxy)oxides for the oxygen evolution reaction. Nat. Energy 5, 222–230 (2020).
Kötz, R., Stucki, S., Scherson, D. & Kolb, D. M. In-situ identification of RuO4 as the corrosion product during oxygen evolution on ruthenium in acid media. J. Electroanal. Chem. 172, 211–219 (1984).
Pfeifer, V. et al. In situ observation of reactive oxygen species forming on oxygen-evolving iridium surfaces. Chem. Sci. 8, 2143–2149 (2017).
Grimaud, A. et al. Activation of surface oxygen sites on an iridium-based model catalyst for the oxygen evolution reaction. Nat. Energy 2, 16189 (2017).
Nong, H. N. et al. Key role of chemistry versus bias in electrocatalytic oxygen evolution. Nature 587, 408–413 (2020).
Mom, R. V. et al. Operando structure-activity-stability relationship of iridium oxides during the oxygen evolution reaction. ACS Catal. 12, 5174–5184 (2022).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Rossmeisl, J., Logadottir, A. & Nørskov, J. K. Electrolysis of water on (oxidized) metal surfaces. Chem. Phys. 319, 178–184 (2005).
Quaino, P., Juarez, F., Santos, E. & Schmickler, W. Volcano plots in hydrogen electrocatalysis—uses and abuses. Beilstein J. Nanotechnol. 5, 846–854 (2014).
Man, I. C. et al. Universality in oxygen evolution electrocatalysis on oxide surfaces. ChemCatChem 3, 1159–1165 (2011).
Pérez-Ramírez, J. & López, N. Strategies to break linear scaling relationships. Nat. Catal. 2, 971–976 (2019).
Suntivich, J. et al. A perovskite oxide optimized for molecular orbital principles. Science 334, 2010–2012 (2011).
Bergmann, A. et al. Reversible amorphization and the catalytically active state of crystalline Co3O4 during oxygen evolution. Nat. Commun. 6, 8625 (2015).
Tung, C. W. et al. Reversible adapting layer produces robust single-crystal electrocatalyst for oxygen evolution. Nat. Commun. 6, 8106 (2015).
Reikowski, F. et al. Operando surface X-ray diffraction studies of structurally defined Co3O4 and CoOOH thin films during oxygen evolution. ACS Catal. 9, 3811–3821 (2019).
Wiegmann, T. et al. Operando identification of the reversible skin layer on Co3O4 as a three-dimensional reaction zone for oxygen evolution. ACS Catal. 12, 3256–3268 (2022).
Lagadec, M. F. & Grimaud, A. Water electrolysers with closed and open electrochemical systems. Nat. Mater. 19, 1140–1150 (2020).
Bernt, M. & Gasteiger, H. A. Influence of ionomer content in IrO2 /TiO2 electrodes on PEM water electrolyzer performance. J. Electrochem. Soc. 163, F3179–F3189 (2016).
Babic, U., Suermann, M., Büchi, F. N., Gubler, L. & Schmidt, T. J. Critical review—identifying critical gaps for polymer electrolyte water electrolysis development. J. Electrochem. Soc. 164, F387–F399 (2017).
Minke, C., Suermann, M., Bensmann, B. & Hanke-Rauschenbach, R. Is iridium demand a potential bottleneck in the realization of large-scale PEM water electrolysis? Int. J. Hydrogen Energy 46, 23581–23590 (2021).
Wilburn, D. R. & Bleiwas, D. I. Platinum-group Metals: World Supply and Demand. US Geological Survey Open-File Report 135–148 (US Geological Survey, 2004).
Hegge, F. et al. Efficient and stable low iridium loaded anodes for PEM water electrolysis made possible by nanofiber interlayers. ACS Appl. Energy Mater. 3, 8276–8284 (2020).
Taie, Z. et al. Pathway to complete energy sector decarbonization with available iridium resources using ultralow loaded water electrolyzers. ACS Appl. Mater. Interfaces 12, 52701–52712 (2020).
Du, N. et al. Anion-exchange membrane water electrolyzers. Chem. Rev. 122, 11830–11895 (2022).
Hugar, K. M., Kostalik, H. A. & Coates, G. W. Imidazolium cations with exceptional alkaline stability: a systematic study of structure-stability relationships. J. Am. Chem. Soc. 137, 8730–8737 (2015).
Peng, X. et al. Using operando techniques to understand and design high performance and stable alkaline membrane fuel cells. Nat. Commun. 11, 3561 (2020).
Ul Hassan, N. et al. Achieving high‐performance and 2,000 h stability in anion exchange membrane fuel cells by manipulating ionomer properties and electrode optimization. Adv. Energy Mater. 10, 2001986 (2020).
Li, D. et al. Durability of anion exchange membrane water electrolyzers. Energy Environ. Sci. 14, 3393–3419 (2021).
Lindquist, G. A. et al. Performance and durability of pure-water-fed anion exchange membrane electrolyzers using baseline materials and operation. ACS Appl. Mater. Interfaces 13, 51917–51924 (2021).
Krivina, R. A. et al. Anode catalysts in anion‐exchange‐membrane electrolysis without supporting electrolyte: conductivity, dynamics and ionomer degradation. Adv. Mater. 34, 2203033 (2022).
Diesendruck, C. E. & Dekel, D. R. Water—a key parameter in the stability of anion exchange membrane fuel cells. Curr. Opin. Electrochem. 9, 173–178 (2018).
Dekel, D. R. et al. Effect of water on the stability of quaternary ammonium groups for anion exchange membrane fuel cell applications. Chem. Mater. 29, 4425–4431 (2017).
Yang, Y. et al. Electrocatalysis in alkaline media and alkaline membrane-based energy technologies. Chem. Rev. 122, 6117–6321 (2022).
Anderson, G. C., Pivovar, B. S. & Alia, S. M. Establishing performance baselines for the oxygen evolution reaction in alkaline electrolytes. J. Electrochem. Soc. 167, 044503 (2020).
Mayerhöfer, B. et al. Electrochemical- and mechanical stability of catalyst layers in anion exchange membrane water electrolysis. Int. J. Hydrogen Energy 47, 4304–4314 (2022).
Wang, J. et al. Poly(aryl piperidinium) membranes and ionomers for hydroxide exchange membrane fuel cells. Nat. Energy 4, 392–398 (2019).
Dekel, D. R. Review of cell performance in anion exchange membrane fuel cells. J. Power Sources 375, 158–169 (2018).
Gottesfeld, S. et al. Anion exchange membrane fuel cells: current status and remaining challenges. J. Power Sources 375, 170–184 (2018).
Exner, K. S., Sohrabnejad-Eskan, I. & Over, H. A universal approach to determine the free energy diagram of an electrocatalytic reaction. ACS Catal. 8, 1864–1879 (2018).
Exner, K. S. Recent progress in the development of screening methods to identify electrode materials for the oxygen evolution reaction. Adv. Funct. Mater. 30, 2005060 (2020).
Moltved, K. A. & Kepp, K. P. The chemical bond between transition metals and oxygen: electronegativity, d-orbital effects, and oxophilicity as descriptors of metal-oxygen interactions. J. Phys. Chem. C 123, 18432–18444 (2019).
Torrie, G. M. & Valleau, J. P. Nonphysical sampling distributions in Monte Carlo free-energy estimation: umbrella sampling. J. Comput. Phys. 23, 187–199 (1977).
Laio, A. & Parrinello, M. Escaping free-energy minima. Proc. Natl Acad. Sci. USA 99, 12562–12566 (2002).
Niu, H., Piaggi, P. M., Invernizzi, M. & Parrinello, M. Molecular dynamics simulations of liquid silica crystallization. Proc. Natl Acad. Sci. USA 115, 5348–5352 (2018).
Sakong, S. & Groß, A. Water structures on a Pt(111) electrode from: ab initio molecular dynamic simulations for a variety of electrochemical conditions. Phys. Chem. Chem. Phys. 22, 10431–10437 (2020).
Sakong, S. & Groß, A. The electric double layer at metal-water interfaces revisited based on a charge polarization scheme. J. Chem. Phys. 149, 084705 (2018).
Kattirtzi, J. A., Limmer, D. T. & Willard, A. P. Microscopic dynamics of charge separation at the aqueous electrochemical interface. Proc. Natl Acad. Sci. USA 114, 13374–13379 (2017).
Limmer, D. T., Willard, A. P., Madden, P. & Chandler, D. Hydration of metal surfaces can be dynamically heterogeneous and hydrophobic. Proc. Natl Acad. Sci. USA 110, 4200–4205 (2013).
Grifoni, E., Piccini, G. & Parrinello, M. Microscopic description of acid-base equilibrium. Proc. Natl Acad. Sci. USA 116, 4054–4057 (2019).
Pinto, L. M. C., Quaino, P., Santos, E. & Schmickler, W. On the electrochemical deposition and dissolution of divalent metal ions. ChemPhysChem 15, 132–138 (2014).
Pinto, L. M. C., Spohr, E., Quaino, P., Santos, E. & Schmickler, W. Why silver deposition is so fast: solving the enigma of metal deposition. Angew. Chem. Int. Ed. 52, 7883–7885 (2013).
Sood, A. et al. Electrochemical ion insertion from the atomic to the device scale. Nat. Rev. Mater. 6, 847–867 (2021).
Fraggedakis, D. et al. Theory of coupled ion-electron transfer kinetics. Electrochim. Acta 367, 137432 (2021).
Trotochaud, L., Young, S. L., Ranney, J. K. & Boettcher, S. W. Nickel-iron oxyhydroxide oxygen-evolution electrocatalysts: the role of intentional and incidental iron incorporation. J. Am. Chem. Soc. 136, 6744–6753 (2014).
Gileadi, E. & Kirowa-Eisner, E. Some observations concerning the Tafel equation and its relevance to charge transfer in corrosion. Corros. Sci. 47, 3068–3085 (2005).
Schmickler, W. & Santos, E. Interfacial Electrochemistry (Springer, 2010).
Gileadi, E. Problems in interfacial electrochemistry that have been swept under the carpet. J. Solid State Electrochem. 15, 1359–1371 (2011).
He, Z. D., Chen, Y. X., Santos, E. & Schmickler, W. The pre-exponential factor in electrochemistry. Angew. Chem. Int. Ed. 57, 7948–7956 (2018).
Guidelli, R. et al. Defining the transfer coefficient in electrochemistry: an assessment (IUPAC Technical Report). Pure Appl. Chem. 86, 245–258 (2014).
Pfeifer, V. et al. Reactive oxygen species in iridium-based OER catalysts. Chem. Sci. 7, 6791–6795 (2016).
Lubitz, W., Reijerse, E. J. & Messinger, J. Solar water-splitting into H2 and O2: design principles of photosystem II and hydrogenases. Energy Environ. Sci. 1, 15–31 (2008).
Haase, F. T. et al. Size effects and active state formation of cobalt oxide nanoparticles during the oxygen evolution reaction. Nat. Energy 7, 765–773 (2022).
Grimaud, A., Hong, W. T. & Tarascon, J. Anionic redox processes for electrochemical devices. Nat. Publ. Gr. 15, 121–126 (2016).
Augustyn, V., Simon, P. & Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 7, 1597–1614 (2014).
Corrigan, D. A. The catalysis of the oxygen evolution reaction by iron impurities in thin film nickel oxide electrodes. J. Electrochem. Soc. 134, 377–384 (1987).
Francàs, L. et al. Spectroelectrochemical study of water oxidation on nickel and iron oxyhydroxide electrocatalysts. Nat. Commun. 10, 5208 (2019).
Grimaud, A. et al. Activating lattice oxygen redox reactions in metal oxides to catalyse oxygen evolution. Nat. Chem. 9, 457–465 (2017).
Surendranath, Y., Kanan, M. W. & Nocera, D. G. Mechanistic studies of the oxygen evolution reaction by a cobalt-phosphate catalyst at neutral pH. J. Am. Chem. Soc. 132, 16501–16509 (2010).
Pearce, P. E. et al. Revealing the reactivity of the iridium trioxide intermediate for the oxygen evolution reaction in acidic media. Chem. Mater. 31, 5845–5855 (2019).
Ryu, J. & Surendranath, Y. Tracking electrical fields at the Pt/H2O interface during hydrogen catalysis. J. Am. Chem. Soc. 141, 15524–15531 (2019).
Boettcher, S. W. & Surendranath, Y. Heterogeneous electrocatalysis goes chemical. Nat. Catal. 4, 4–5 (2021).
Boettcher, S. W. et al. Potentially confusing: potentials in electrochemistry. ACS Energy Lett. 6, 261–266 (2021).
Bergmann, A. et al. Unified structural motifs of the catalytically active state of Co(oxyhydr)oxides during the electrochemical oxygen evolution reaction. Nat. Catal. 1, 711–719 (2018).
Friebel, D. et al. Identification of highly active Fe sites in (Ni, Fe)OOH for electrocatalytic water splitting. J. Am. Chem. Soc. 137, 1305–1313 (2015).
Demourgues-Guerlou, L., Braconnier, J. J. & Delmas, C. Iron-substituted nickel oxyhydroxides and hydroxides obtained by chimie douce. J. Solid State Chem. 104, 359–367 (1993).
Burke, M. S., Kast, M. G., Trotochaud, L., Smith, A. M. & Shannon, W. B. Cobalt-iron (oxy)hydroxide oxygen evolution electrocatalysts: the role of structure and composition on activity, stability and mechanism. J. Am. Chem. Soc. 137, 3638–3648 (2015).
Krivina, R. A. et al. Oxygen electrocatalysis on mixed-metal oxides/oxyhydroxides: from fundamentals to membrane electrolyzer technology. Acc. Mater. Res. 2, 548–558 (2021).
Zhou, Y. & López, N. The role of Fe species on NiOOH in oxygen evolution reactions. ACS Catal. 10, 6254–6261 (2020).
Zeradjanin, A. R., Menzel, N., Strasser, P. & Schuhmann, W. Role of water in the chlorine evolution reaction at RuO2-based electrodes-understanding electrocatalysis as a resonance phenomenon. ChemSusChem 5, 1897–1904 (2012).
Trasatti, S. Electrocatalysis in the anodic evolution of oxygen and chlorine. Electrochim. Acta 29, 1503–1512 (1984).
Diaz-Morales, O., Ferrus-Suspedra, D. & Koper, M. T. M. The importance of nickel oxyhydroxide deprotonation on its activity towards electrochemical water oxidation. Chem. Sci. 7, 2639–2645 (2016).
Garcia, A. C., Touzalin, T., Nieuwland, C., Perini, N. & Koper, M. T. M. Enhancement of oxygen evolution activity of nickel oxyhydroxide by electrolyte alkali cations. Angew. Chem. Int. Ed. 58, 12999–13003 (2019).
Dette, C., Hurst, M. R., Deng, J., Nellist, M. R. & Boettcher, S. W. Structural evolution of metal (oxy)hydroxide nanosheets during the oxygen evolution reaction. ACS Appl. Mater. Interfaces 11, 5590–5594 (2019).
Deng, J. et al. Morphology dynamics of single-layered Ni(OH)2/NiOOH nanosheets and subsequent Fe incorporation studied by in situ electrochemical atomic force microscopy. Nano Lett. 17, 6922–6926 (2017).
Liu, Z., Ma, R., Osada, M., Takada, K. & Sasaki, T. Selective and controlled synthesis of α- and β-cobalt hydroxides in highly developed hexagonal platelets. J. Am. Chem. Soc. 127, 13869–13874 (2005).
Mefford, J. T. et al. Correlative operando microscopy of oxygen evolution electrocatalysts. Nature 593, 67–73 (2021).
Norman, N. C. & Pringle, P. G. In defence of oxidation states. Dalt. Trans. 51, 400–410 (2022).
Karen, P., McArdle, P. & Takats, J. Toward a comprehensive definition of oxidation state (IUPAC Technical Report). Pure Appl. Chem. 86, 1017–1081 (2014).
Favaro, M. et al. Understanding the oxygen evolution reaction mechanism on CoOx using operando ambient-pressure X-ray photoelectron spectroscopy. J. Am. Chem. Soc. 139, 8960–8970 (2017).
Yang, J. et al. A multifunctional biphasic water splitting catalyst tailored for integration with high-performance semiconductor photoanodes. Nat. Mater. 16, 335–341 (2017).
Schlögl, R. Heterogeneous catalysis. Angew. Chem. Int. Ed. 54, 3465–3520 (2015).
Zhang, R. et al. Importance of water structure and catalyst-electrolyte interface on the design of water splitting catalysts. Chem. Mater. 31, 8248–8259 (2019).
Gonella, G. et al. Water at charged interfaces. Nat. Rev. Chem. 5, 466–485 (2021).
Oener, S. Z., Foster, M. J. & Boettcher, S. W. Accelerating water dissociation in bipolar membranes and for electrocatalysis. Science 369, 1099–1103 (2020).
Tang, B. Y., Bisbey, R. P., Lodaya, K. M., Toh, W. L. & Surendranath, Y. Reaction environment impacts charge transfer but not chemical reaction steps in hydrogen evolution catalysis. Nat. Catal. 6, 339–350 (2023).
Löffler, T., Ludwig, A., Rossmeisl, J. & Schuhmann, W. What makes high-entropy alloys exceptional electrocatalysts? Angew. Chem. Int. Ed. https://doi.org/10.1002/anie.202109212 (2021).
Batchelor, T. A. A. et al. Complex‐solid‐solution electrocatalyst discovery by computational prediction and high‐throughput experimentation. Angew. Chem. Int. Ed. 60, 6932–6937 (2021).
Svane, K. L. & Rossmeisl, J. Theoretical optimization of compositions of high-entropy oxides for the oxygen evolution reaction. Angew. Chem. Int. Ed. 61 https://doi.org/10.1002/anie.202201146 (2022).
Timoshenko, J. & Roldan Cuenya, B. In situ/operando electrocatalyst characterization by X-ray absorption spectroscopy. Chem. Rev. 121, 882–961 (2021).
Scheffler, M. et al. FAIR data enabling new horizons for materials research. Nature 604, 635–642 (2022).
May, K. J. et al. Influence of oxygen evolution during water oxidation on the surface of perovskite oxide catalysts. J. Phys. Chem. Lett. 3, 3264–3270 (2012).
Risch, M. Perovskite electrocatalysts for the oxygen reduction reaction in alkaline media. Catalysts 7, 154 (2017).
Risch, M. et al. Structural changes of cobalt-based perovskites upon water oxidation investigated by EXAFS. J. Phys. Chem. C 117, 8628–8635 (2013).
Baeumer, C. et al. Tuning electrochemically driven surface transformation in atomically flat LaNiO3 thin films for enhanced water electrolysis. Nat. Mater. 20, 674–682 (2021).
Thorarinsdottir, A. E., Veroneau, S. S. & Nocera, D. G. Self-healing oxygen evolution catalysts. Nat. Commun. 13, 1243 (2022).
Timoshenko, J. et al. Steering the structure and selectivity of CO2 electroreduction catalysts by potential pulses. Nat. Catal. 5, 259–267 (2022).
Cherevko, S. et al. Dissolution of noble metals during oxygen evolution in acidic media. ChemCatChem 6, 2219–2223 (2014).
Nong, H. N. et al. A unique oxygen ligand environment facilitates water oxidation in hole-doped IrNiOX core–shell electrocatalysts. Nat. Catal. 1, 841–851 (2018).
Yang, C. et al. Cation insertion to break the activity/stability relationship for highly active oxygen evolution reaction catalyst. Nat. Commun. 11, 1378 (2020).
Zeradjanin, A. R., Masa, J., Spanos, I. & Schlögl, R. Activity and stability of oxides during oxygen evolution reaction‐from mechanistic controversies toward relevant electrocatalytic descriptors. Front. Energy Res. 8, 1–17 (2021).
Martelli, G. N., Ornelas, R. & Faita, G. Deactivation mechanisms of oxygen evolving anodes at high current densities. Electrochim. Acta 39, 1551–1558 (1994).
Antonyshyn, I. et al. Al2Pt for oxygen evolution in water splitting: a strategy for creating multifunctionality in electrocatalysis. Angew. Chem. Int. Ed. 59, 16770–16776 (2020).
Mondschein, J. S. et al. Intermetallic Ni2Ta electrocatalyst for the oxygen evolution reaction in highly acidic electrolytes. Inorg. Chem. 57, 6010–6015 (2018).
Barrios Jimenez, A. M. et al. Hf2B2Ir5: a self-optimizing catalyst for the oxygen evolution reaction. ACS Appl. Energy Mater. 3, 11042–11052 (2020).
IRENA. Green Hydrogen Cost Reduction (IRENA, 2020); https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2020/Dec/IRENA_Green_hydrogen_cost_2020.pdf
Holst, M., Aschbrenner, S., Smolinka, T., Voglstätter, C. & Grimm, G. Cost Forecast for Low Temperature Electrolysis - Technology Driven Bottom-up Prognosis for PEM and Alkaline Water Electrolysis Systems (Fraunhofer Institut, 2021); https://doi.org/10.24406/publica-1318
Schäfer, H. & Chatenet, M. Steel: the resurrection of a forgotten water-splitting catalyst. ACS Energy Lett. 3, 574–591 (2018).
Schäfer, H. et al. Steel-based electrocatalysts for efficient and durable oxygen evolution in acidic media. Catal. Sci. Technol. 8, 2104–2116 (2018).
Acknowledgements
This work was funded by the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung, BMBF) under grant no. 03EW0015B (CATLAB).
Author information
Authors and Affiliations
Contributions
S.Z.O. wrote the manuscript, with help from A.B. and B.R.C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks the anonymous reviewers for their contribution to the peer review of this work. Principal Handling Editor: Alexandra Groves, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oener, S.Z., Bergmann, A. & Cuenya, B.R. Designing active oxides for a durable oxygen evolution reaction. Nat. Synth 2, 817–827 (2023). https://doi.org/10.1038/s44160-023-00376-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-023-00376-6
This article is cited by
-
Ion solvation kinetics in bipolar membranes and at electrolyte–metal interfaces
Nature Energy (2024)
-
High-throughput electrochemical strategy for synthesis of iron-based nanostructures for electrocatalytic water splitting
Journal of Materials Science (2024)