Abstract
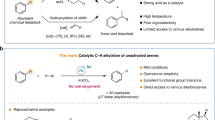
The development of practical approaches for the selective functionalization of strong, neutral C(sp3)–H bonds, such as those in petroleum-derived hydrocarbons, is of general interest but remains a remarkable challenge in synthetic chemistry. We here report a convenient approach that employs allyl bromides as the reagents and sodium fluoride as an activator in photochemical processes. Diverse C(sp3)–H functionalizations of alkanes, cycloalkanes and other relatively unreactive substances were enabled by using a stoichiometric or catalytic amount of allyl bromides as initiators in the presence of NaF, which furnished various allylated, heteroarylated, alkylated, hydrazinated and aminated products in good yields with high chemoselectivity and site selectivity. Binary NaF–allyl bromide adducts generated in situ appear to play essential roles in the reaction as light-active species, initiators for radical-mediated C–H cleavage and potential functionalization reagents. We expect that this transition-metal- and photosensitizer-free strategy will offer new opportunities for the C–H diversification of hydrocarbon feedstocks and the late-stage modification of lead compounds.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All relevant data supporting the findings of this study, including experimental procedures, compound characterization, computational study details, NMR spectra and other spectroscopic analysis, are available within the article and its Supplementary Information.
References
Labinger, J. A. & Bercaw, J. E. Understanding and exploiting C–H bond activation. Nature 417, 507–514 (2002).
Davies, H. M. L. & Liao, K. Dirhodium tetracarboxylates as catalysts for selective intermolecular C–H functionalization. Nat. Rev. Chem. 3, 347–360 (2019).
Kariofillis, S. K. & Doyle, A. G. Synthetic and mechanistic implications of chlorine photoelimination in nickel/photoredox C(sp3)–H cross-coupling. Acc. Chem. Res. 54, 988–1000 (2021).
Fazekas, T. J. et al. Diversification of aliphatic C–H bonds in small molecules and polyolefins through radical chain transfer. Science 375, 545–550 (2022).
Le, C., Liang, Y., Evans, R. W., Li, X. & MacMillan, D. W. C. Selective sp3 C-H alkylation via polarity-matchbased cross-coupling. Nature 547, 79–83 (2017).
Capaldo, L., Ravelli, D. & Fagnori, M. Direct photocatalyzed hydrogen atom transfer (HAT) for aliphatic C–H bonds elaboration. Chem. Rev. 122, 1875–1924 (2022).
Goldberg, K. I. & Goldman, A. S. Large-scale selective functionalization of alkanes. Acc. Chem. Res. 50, 620–626 (2017).
Feng, K. et al. Late-stage oxidative C(sp3)–H methylation. Nature 580, 621–627 (2020).
Perry, I. B. et al. Direct arylation of strong aliphatic C–H bonds. Nature 560, 70–75 (2018).
Hu, A., Guo, J.-J., Pan, H. & Zuo, Z. Selective functionalization of methane, ethane, and higher alkanes by cerium photocatalysis. Science 361, 668–672 (2018).
Sharma, A. & Hartwig, J. F. Metal-catalysed azidation of tertiary C–H bonds suitable for late-stage functionalization. Nature 517, 600–604 (2015).
Huang, H.-M., Bellotti, P., Chen, P.-P., Houk, K. N. & Glorius, F. Allylic C(sp3)–H arylation of olefins via ternary catalysis. Nat. Synth. 1, 59–68 (2022).
Mukherjee, S., Maji, B., Tlahuext-Aca, A. & Glorius, F. Visible-light-promoted activation of unactivated C(sp3)–H bonds and their selective trifluoromethylthiolation. J. Am. Chem. Soc. 138, 16200–16203 (2016).
Tsuji, T. et al. α-Amino acid and peptide synthesis using catalytic cross-dehydrogenative coupling. Nat. Synth. 1, 304–312 (2022).
Sarver, P. J. et al. The merger of decatungstate and copper catalysis to enable aliphatic C(sp3)–H trifluoromethylation. Nat. Chem. 12, 459–467 (2020).
Chu, J. C. K. & Rovis, T. Amide-directed photoredox-catalysed C–C bond formation at unactivated sp3 C–H bonds. Nature 539, 272–275 (2016).
Liao, K., Negretti, S., Musaev, D. G., Bacsa, J. & Davies, H. M. L. Site-selective and stereoselective functionalization of unactivated C–H bonds. Nature 533, 230–234 (2016).
Liao, K. et al. Site-selective and stereoselective functionalization of non-activated tertiary C–H bonds. Nature 551, 609–613 (2017).
Shu, C., Noble, A. & Aggarwal, V. K. Metal-free photoinduced C(sp3)–H borylation of alkanes. Nature 586, 714–719 (2020).
Rohe, S., Morris, A. O., McCallum, A. O. & Barriault, L. Hydrogen atom transfer reactions via photoredox catalyzed chlorine atom generation. Angew. Chem. Int. Ed. 57, 15664–15669 (2018).
Jia, P. et al. Light-promoted bromine-radical-mediated selective alkylation and amination of unactivated C(sp3)–H bonds. Chem 6, 1766–1776 (2020).
Silvi, M. & Melchiorre, P. Enhancing the potential of enantioselective organocatalysis with light. Nature 554, 41–49 (2018).
Strieth-Kalthoff, F. & Glorius, F. Triplet energy transfer photocatalysis: unlocking the next level. Chem 6, 1888–1903 (2020).
Fu, M.-C., Shang, R., Zhao, B., Wang, B. & Fu, Y. Photocatalytic decarboxylative alkylations mediated by triphenylphosphine and sodium iodide. Science 363, 1429–1434 (2019).
Saux, E. L., Zanini, M. & Melchiorre, P. Photochemical organocatalytic benzylation of allylic C–H bonds. J. Am. Chem. Soc. 144, 1113–1118 (2022).
Li, Y., Lei, M. & Gong, L. Photocatalytic regio- and stereoselective C(sp3)–H functionalization of benzylic and allylic hydrocarbons as well as unactivated alkanes. Nat. Catal. 2, 1016–1026 (2019).
Cao, S., Hong, W., Ye, Z. & Gong, L. Photocatalytic three-component asymmetric sulfonylation via direct C(sp3)–H functionalization. Nat. Commun. 12, 2377 (2021).
Li, Y. et al. Copper(II)-catalyzed asymmetric photoredox reactions: enantioselective alkylation of imines driven by visible light. J. Am. Chem. Soc. 140, 15850–15858 (2018).
Li, Y. et al. Organophotocatalytic selective deuterodehalogenation of aryl or alkyl chlorides. Nat. Commun. 12, 2894 (2021).
Cao, S. et al. Nickel-catalyzed regiodivergent asymmetric cycloadditions of α,β-unsaturated carbonyl compounds. CCS Chem. 3, 3329–3340 (2021).
Zhang, S. et al. Photocatalyzed site-selective C(sp3)–H sulfonylation of toluene derivatives and cycloalkanes with inorganic sulfinates. Chin. J. Catal. 43, 564–570 (2022).
Katsuto, H., Okamoto, R., Sumi, T. & Koga, K. Ion size dependences of the salting-out effect: reversed order of sodium and lithium ions. J. Phys. Chem. B 125, 6296–6305 (2021).
Daniele, P. G., Rigano, C. & Sammartano, S. Studies on sulphate complexes. Part I. Potentiometric investigation of Li+, Na+, K+, Rb+ and Cs+ complexes at 37 °C and 0.03 ⩽ I ⩽ 0.5. Inorg. Chim. Acta 63, 267–272 (1982).
Wang, L.-T. & Su, T.-M. Molecular complexes between sodium and carbonyl compounds: photoionization and ab initio molecular orbital studies. J. Phys. Chem. A 104, 10825–10833 (2000).
Zhang, G.-Z., Fu, M.-C., Zhao, B. & Shang, R. Photocatalytic decarboxylative alkylations of C(sp3)–H and C(sp2)–H bonds enabled by ammonium iodide in amide solvent. Sci. China Chem. 64, 439–444 (2021).
Struss, J. A., Sadeghipour, M. & Tanko, J. M. Radical additions to allyl bromides. A synthetically useful, ‘tin-free’ method for carbon–carbon bond formation. Tetrahedron Lett. 50, 2119–2120 (2009).
Ghosh, M. et al. Formation of a room temperature stable FeV(O) complex: reactivity toward unactivated C–H bonds. J. Am. Chem. Soc. 136, 9524–9527 (2014).
Fu, J., Ren, Z., Bacsa, J., Musaev, D. G. & Davies, H. M. L. Desymmetrization of cyclohexanes by site- and stereoselective C–H functionalization. Nature 564, 395–399 (2018).
Heitz, D. R., Rizwan, K. & Molander, G. A. Visible-light-mediated alkenylation, allylation, and cyanation of potassium alkyltrifluoroborates with organic photoredox catalysts. J. Org. Chem. 81, 7308–7313 (2016).
Chen, C.-H. & Gabbaï, F. P. Fluoride anion complexation by a triptycene-based distiborane: taking advantage of a weak but observable C−H⋅⋅⋅F interaction. Angew. Chem. Int. Ed. 56, 1799–1804 (2017).
Lu, T. & Chen, F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Zhao, Y., Bordwell, F. G., Cheng, J.-P. & Wang, D. Equilibrium acidities and homolytic bond dissociation energies (BDEs) of the acidic H−N bonds in hydrazines and hydrazides. J. Am. Chem. Soc. 119, 9125–9129 (1997).
Kato, T. & Maruoka, K. Design of bowl-shaped N-hydroxyimide derivatives as new organoradical catalysts for site-selective C(sp3)–H bond functionalization reactions. Angew. Chem. Int. Ed. 59, 14261–14264 (2020).
Tlahuext-Aca, A., Garza-Sanchez, R. A., Schäfer, M. & Glorius, F. Visible-light-mediated synthesis of ketones by the oxidative alkylation of styrenes. Org. Lett. 20, 1546–1549 (2018).
Wegner, E. E. & Adamson, A. W. Photochemistry of complex ions. III. Absolute quantum yields for the photolysis of some aqueous chromium(III) complexes. Chemical actinometry in the long wavelength visible region. J. Am. Chem. Soc. 88, 394–404 (1966).
Tlahuext-Aca, A., Candish, L., Garza-Sanchez, R. A. & Glorius, F. Decarboxylative olefination of activated aliphatic acids enabled by dual organophotoredox/copper catalysis. ACS Catal. 8, 1715–1719 (2018).
Buzzetti, L., Crisenza, G. E. M. & Melchiorre, P. Mechanistic studies in photocatalysis. Angew. Chem. Int. Ed. 58, 3730–3747 (2019).
Lee, W., Jung, S., Kim, M. & Hong, S. Site-selective direct C–H pyridylation of unactivated alkanes by triplet excited anthraquinone. J. Am. Chem. Soc. 143, 3003–3012 (2021).
Acknowledgements
We gratefully acknowledge funding from the National Natural Science Foundation of China (grant nos. 22071209 and 22071206), the National Youth Talent Support Program, the Natural Science Foundation of Fujian Province of China (grant no. 2017J06006) and the Fundamental Research Funds for the Central Universities (grant no. 20720190048).
Author information
Authors and Affiliations
Contributions
L.G. conceived and designed the project. Z.Y., Y.Y., Y.C. and S.S. conducted the experiments, Z.Y. designed and performed the DFT calculations, and with L.G., analysed and interpreted the experimental data. L.G. and Y.-M.L. prepared the manuscript, and Z.Y. and Y.Y. prepared the Supplementary Information.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Rui Shang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Peter Seavill, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ye, Z., Yu, Y., Lin, YM. et al. Photochemical diversification of strong C(sp3)–H bonds enabled by allyl bromide and sodium fluoride. Nat. Synth 2, 766–777 (2023). https://doi.org/10.1038/s44160-023-00291-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-023-00291-w
This article is cited by
-
Cu(II)-Mediated direct 18F-dehydrofluorination of phosphine oxides in high molar activity
EJNMMI Radiopharmacy and Chemistry (2024)
-
Visible light-triggered selective C(sp2)-H/C(sp3)-H coupling of benzenes with aliphatic hydrocarbons
Nature Communications (2023)